Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Question 1: As the hydrogen gas, H2, is passed across the heated nuclear reactor, it can dissociate into atomic hydrogen, H. This process is represented
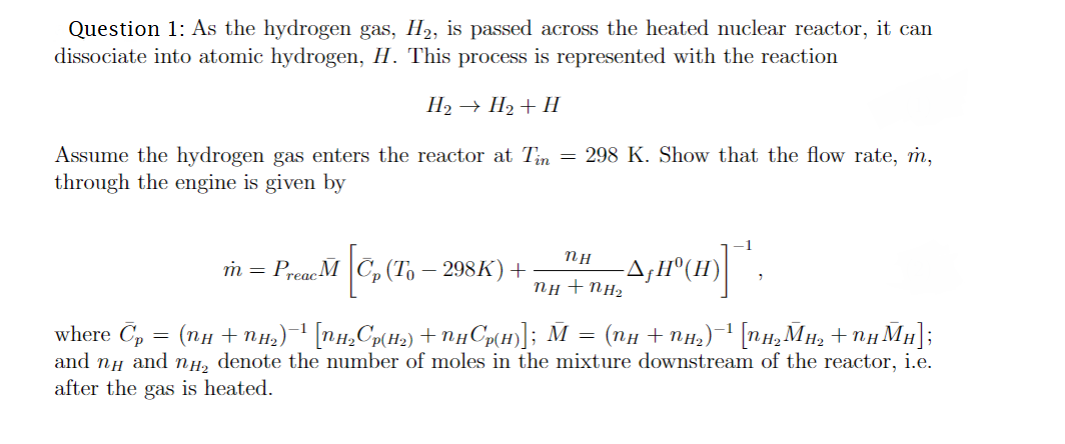 Question 1: As the hydrogen gas, H2, is passed across the heated nuclear reactor, it can dissociate into atomic hydrogen, H. This process is represented with the reaction H2H2+H Assume the hydrogen gas enters the reactor at Tin=298K. Show that the flow rate, m, through the engine is given by m=PreacM[Cp(T0298K)+nH+nH2nHfH0(H)]1, where Cp=(nH+nH2)1[nH2Cp(H2)+nHCp(H)];M=(nH+nH2)1[nH2MH2+nHMH]; and nH and nH2 denote the number of moles in the mixture downstream of the reactor, i.e. after the gas is heated
Question 1: As the hydrogen gas, H2, is passed across the heated nuclear reactor, it can dissociate into atomic hydrogen, H. This process is represented with the reaction H2H2+H Assume the hydrogen gas enters the reactor at Tin=298K. Show that the flow rate, m, through the engine is given by m=PreacM[Cp(T0298K)+nH+nH2nHfH0(H)]1, where Cp=(nH+nH2)1[nH2Cp(H2)+nHCp(H)];M=(nH+nH2)1[nH2MH2+nHMH]; and nH and nH2 denote the number of moles in the mixture downstream of the reactor, i.e. after the gas is heated Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


