Question
Question 1 Boyle's Law states that at constant temperature and number of moles of gas, the pressure is inversely proportional to the volume. What
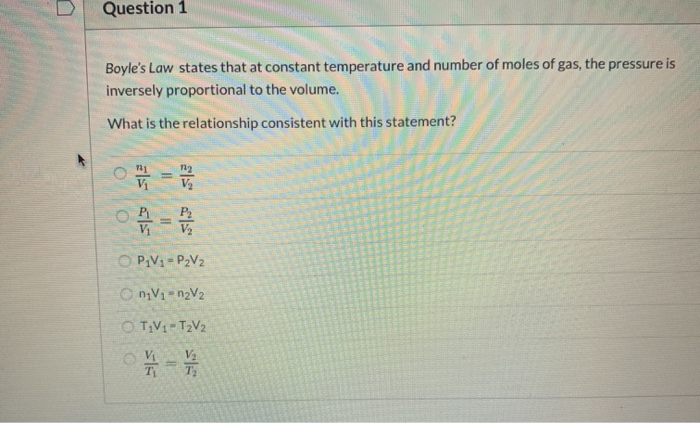
Question 1 Boyle's Law states that at constant temperature and number of moles of gas, the pressure is inversely proportional to the volume. What is the relationship consistent with this statement? %3D V2 %3D O PV1= P2V2 O nV1- n2V2 O TV1- T2V2 O Vi V2
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Answer The relationship consistent with this statement is P1V1 P2V2 Explanation Boyles La...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Calculus
Authors: Dale Varberg, Edwin J. Purcell, Steven E. Rigdon
9th edition
131429248, 978-0131429246
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



