Answered step by step
Verified Expert Solution
Question
1 Approved Answer
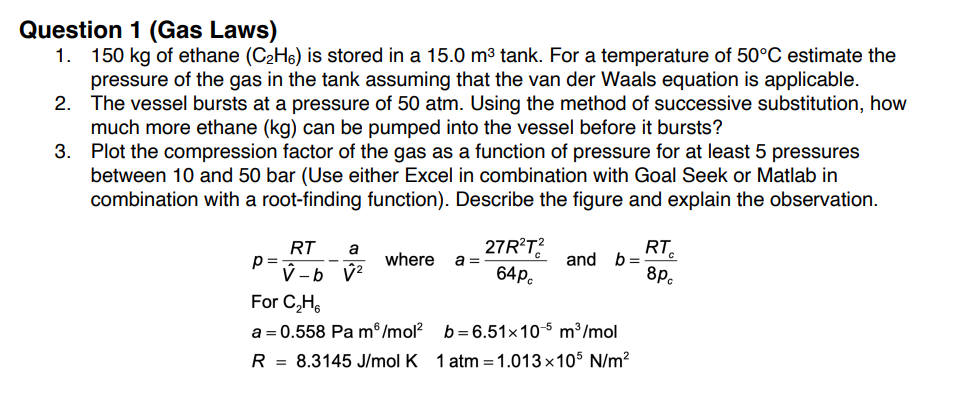
Question 1 ( Gas Laws ) 1 5 0 k g of ethane ( C 2 H 6 ) is stored in a 1 5
Question Gas Laws
of ethane is stored in a tank For a temperature of estimate the
pressure of the gas in the tank assuming that the van der Waals equation is applicable.
The vessel bursts at a pressure of atm. Using the method of successive substitution, how
much more ethane can be pumped into the vessel before it bursts?
Plot the compression factor of the gas as a function of pressure for at least pressures
between and bar Use either Excel in combination with Goal Seek or Matlab in
combination with a rootfinding function Describe the figure and explain the observation.
where and
For
atm
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


