Answered step by step
Verified Expert Solution
Question
1 Approved Answer
QUESTION 1 In the Boyle's Law experiment, you studied how the pressure of the gas sample changed when the volume of the sample changed.
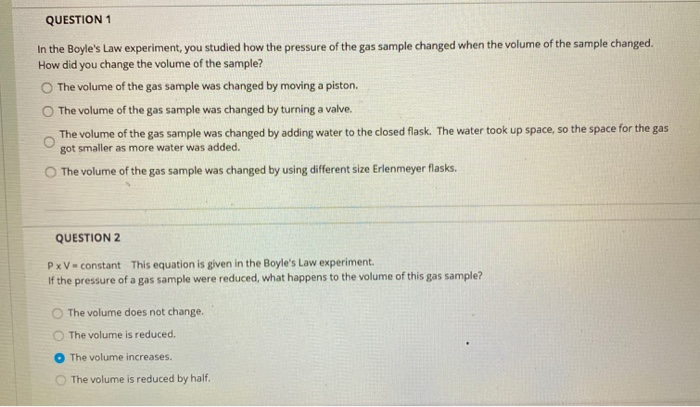
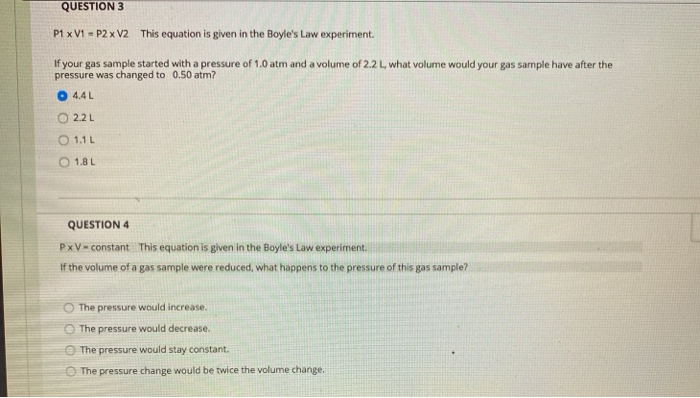

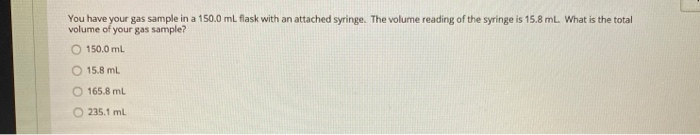
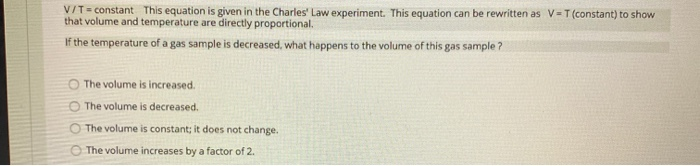
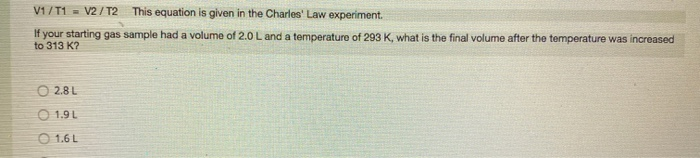
QUESTION 1 In the Boyle's Law experiment, you studied how the pressure of the gas sample changed when the volume of the sample changed. How did you change the volume of the sample? O The volume of the gas sample was changed by moving a piston. The volume of the gas sample was changed by turning a valve. The volume of the gas sample was changed by adding water to the closed flask. The water took up space, so the space for the gas got smaller as more water was added. The volume of the gas sample was changed by using different size Erlenmeyer flasks. QUESTION 2 PxV constant This equation is given in the Boyle's Law experiment. If the pressure of a gas sample were reduced, what happens to the volume of this gas sample? The volume does not change. The volume is reduced. The volume increases. The volume is reduced by half. QUESTION 3 P1 x V1 = P2 x V2 This equation is given in the Boyle's Law experiment. If your gas sample started with a pressure of 1.0 atm and a volume of 2.2 L, what volume would your gas sample have after the pressure was changed to 0.50 atm? 4.4 L 2.2 L 0111 O18L QUESTION 4 PxV-constant This equation is given in the Boyle's Law experiment. If the volume of a gas sample were reduced, what happens to the pressure of this gas sample? The pressure would increase. The pressure would decrease. The pressure would stay constant. The pressure change would be twice the volume change. Charles' Law states that temperature and volume are directly proportional. If the temperature of a gas sample is doubled, what happens to the volume of the gas sample? (For example, the temperature is increased from 300 K to 600 K). O The volume of the gas sample is also doubled. The volume of the gas sample is decreased by a factor of 3. The volume of the gas sample does not change. The volume of the gas sample decreases by a factor of 4. You have your gas sample in a 150.0 mL flask with an attached syringe. The volume reading of the syringe is 15.8 mL. What is the total volume of your gas sample? O 150.0 mL 15.8 mL O 165.8 mL 235.1 mL V/T=constant This equation is given in the Charles' Law experiment. This equation can be rewritten as V-T (constant) to show that volume and temperature are directly proportional. If the temperature of a gas sample is decreased, what happens to the volume of this gas sample? The volume is increased. The volume is decreased. The volume is constant; it does not change. The volume increases by a factor of 2. V1/T1= V2/T2 This equation is given in the Charles' Law experiment. If your starting gas sample had a volume of 2.0 L and a temperature of 293 K, what is the final volume after the temperature was increased to 313 K? 2.8L 1.9 L 1.6 L
Step by Step Solution
★★★★★
3.54 Rating (171 Votes )
There are 3 Steps involved in it
Step: 1
The detailed answer for the above question is provided below Q1 Option A is correct The volume of ga...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


