Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Question 1 KOH+HPO4 -> K3PO4+HO Is it Arrhenius, Bronsted-lowry or Arrherlius & Bronsted-lowry? Ob Oc Od e Of O a Ob Oc Arrhenius HO
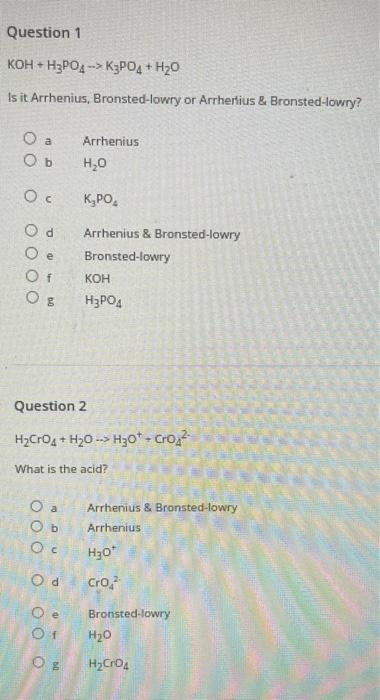
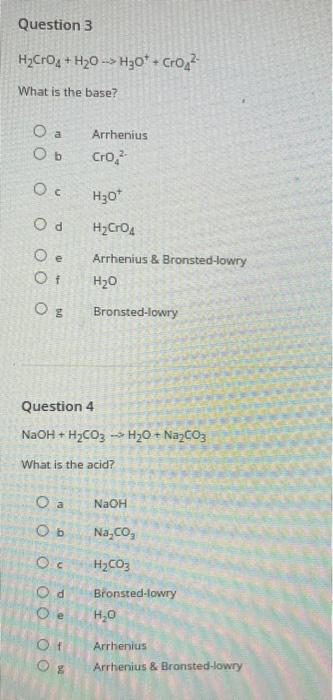

Question 1 KOH+HPO4 -> K3PO4+HO Is it Arrhenius, Bronsted-lowry or Arrherlius & Bronsted-lowry? Ob Oc Od e Of O a Ob Oc Arrhenius HO Question 2 HCrO4 + HO -> HO + Cro What is the acid? Of KPO Arrhenius & Bronsted-lowry Bronsted-lowry O E KOH HPO4 Od Cro Arrhenius & Bronsted-lowry Arrhenius HO* Bronsted-lowry HO HCrO4 Question 3 HCrO4 + H0 H30* + Cro What is the base? O a Ob Oc Od Oe Of OO Question 4 NaOH + HCO3 -> HO + NaCO3 What is the acid? OO Arrhenius CrO, O a O b NaCO Oc HCO3 d H30* HCrO4 Arrhenius & Bronsted-lowry HO O Bronsted-lowry NaOH Bronsted-lowry HO Arrhenius Arrhenius & Bronsted-lowry Question 5 What are the products of the following reaction: HCI + RbOH O a Ob Oc Od Question 6 NaOH + HCO3 -> HO + NaCO3 What is the base? O a Ob Od Oe Of RbO + HCl HOCI + Rb HO + RbCl RbCI+ OH Submit Arrhenius NaCO, HO HCO3 NaOH Arrhenius & Bronsted-lowry Bronsted-lowry
Step by Step Solution
★★★★★
3.43 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1
The detailed answer for the above question is provided below Question 1 The corre...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


