Answered step by step
Verified Expert Solution
Question
1 Approved Answer
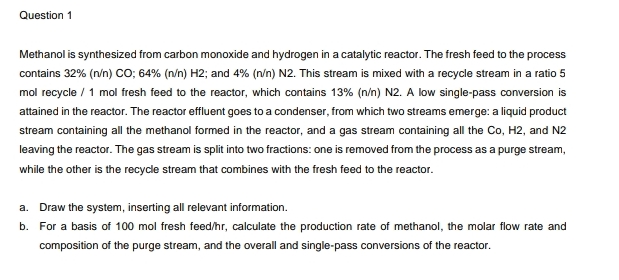
Question 1 Methanol is synthesized from carbon monoxide and hydrogen in a catalytic reactor. The fresh feed to the process contains 3 2 % (
Question
Methanol is synthesized from carbon monoxide and hydrogen in a catalytic reactor. The fresh feed to the process contains ;; and N This stream is mixed with a recycle stream in a ratio mol recycle mol fresh feed to the reactor, which contains A low singlepass conversion is attained in the reactor. The reactor effluent goes to a condenser, from which two streams emerge: a liquid product stream containing all the methanol formed in the reactor, and a gas stream containing all the Co and N leaving the reactor. The gas stream is split into two fractions: one is removed from the process as a purge stream, while the other is the recycle stream that combines with the fresh feed to the reactor.
a Draw the system, inserting all relevant information.
b For a basis of mol fresh feed calculate the production rate of methanol, the molar flow rate and composition of the purge stream, and the overall and singlepass conversions of the reactor.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


