Answered step by step
Verified Expert Solution
Question
1 Approved Answer
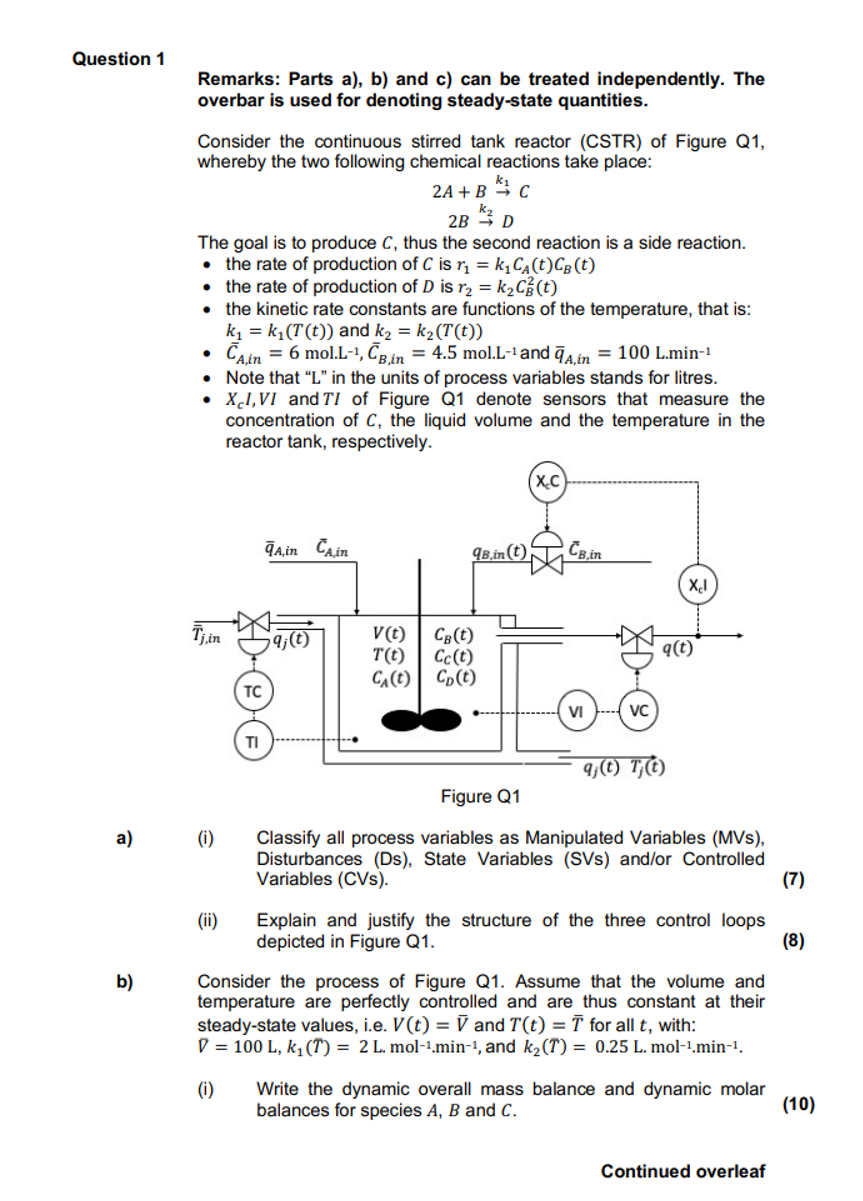
Question 1 Remarks: Parts a ) , b ) and c ) can be treated independently. The overbar is used for denoting steady - state
Question
Remarks: Parts a b and c can be treated independently. The
overbar is used for denoting steadystate quantities.
Consider the continuous stirred tank reactor CSTR of Figure Q
whereby the two following chemical reactions take place:
The goal is to produce thus the second reaction is a side reaction.
the rate of production of is
the rate of production of is
the kinetic rate constants are functions of the temperature, that is:
and
mol.mol. and
Note that L in the units of process variables stands for litres.
I, and of Figure Q denote sensors that measure the
concentration of the liquid volume and the temperature in the
reactor tank, respectively.
riguie wi
a
i Classify all process variables as Manipulated Variables MVs
Disturbances Ds State Variables SVs andor Controlled
Variables CVs
ii Explain and justify the structure of the three control loops
depicted in Figure Q
b Consider the process of Figure Q Assume that the volume and
temperature are perfectly controlled and are thus constant at their
steadystate values, ie and for all with:
and
i Write the dynamic overall mass balance and dynamic molar
balances for species and
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


