Answered step by step
Verified Expert Solution
Question
1 Approved Answer
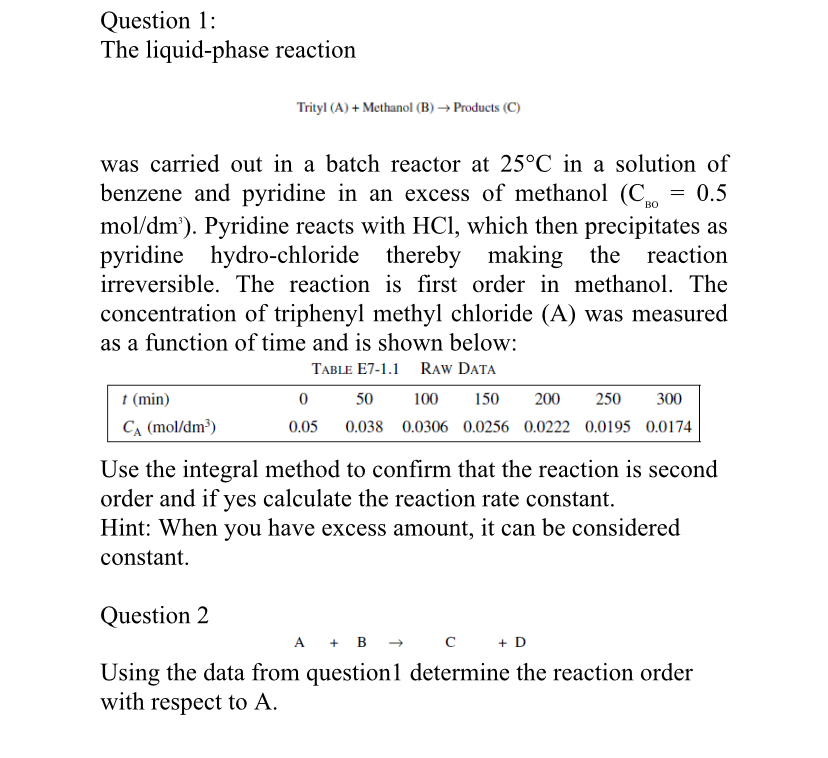
Question 1 : The liquid - phase reaction Trityl ( A ) + Methanol ( B ) Products ( C ) was carried out in
Question :
The liquidphase reaction
Trityl A Methanol B Products C
was carried out in a batch reactor at in a solution of benzene and pyridine in an excess of methanol : Pyridine reacts with which then precipitates as pyridine hydrochloride thereby making the reaction irreversible. The reaction is first order in methanol. The concentration of triphenyl methyl chloride A was measured as a function of time and is shown below:
tableTABLE ERAW DATA,,,,,,
Use the integral method to confirm that the reaction is second order and if yes calculate the reaction rate constant.
Hint: When you have excess amount, it can be considered constant.
Question
Using the data from question determine the reaction order with respect to
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


