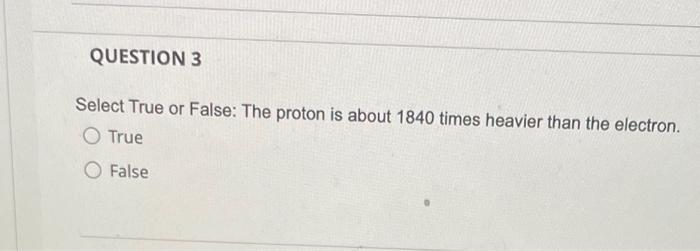
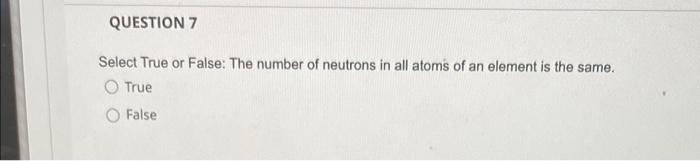
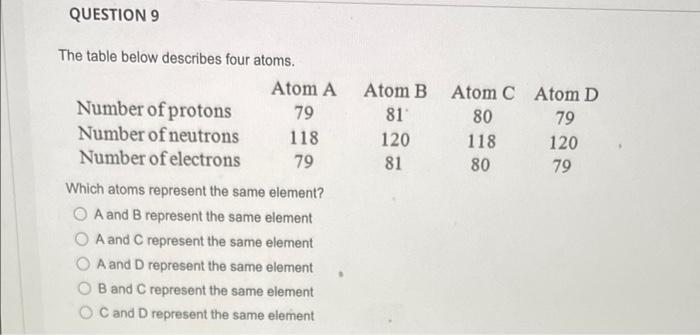
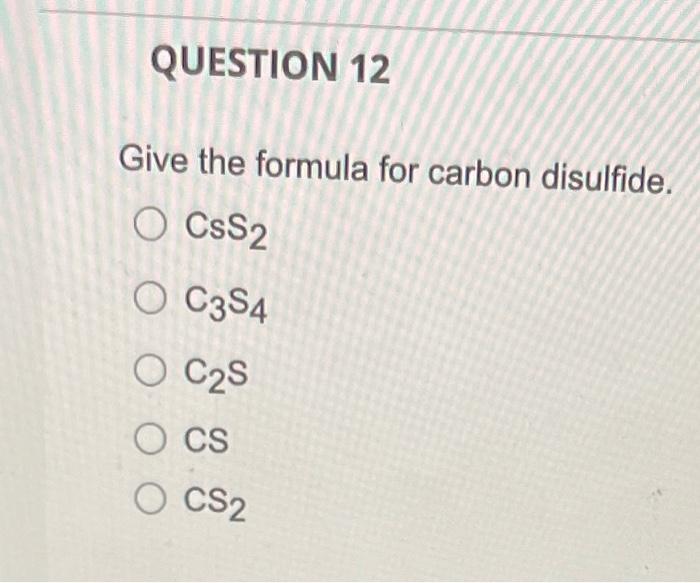
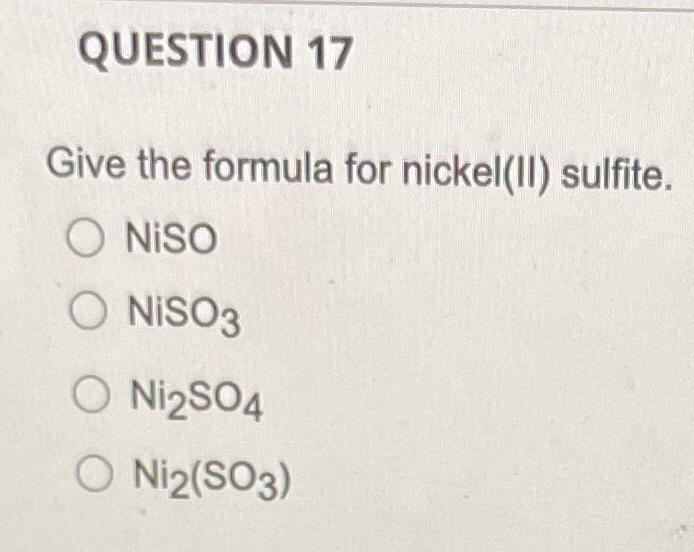
QUESTION 1 What is the law of conservation of mass? Gravity and mass have the same meaning. Matter can be neither created nor destroyed. Mass can never be changed to energy. Mass and volume will always be equal. Mass can be destroyed but only when it is conserved. QUESTION 2 Which listing provides the three common types of radiation that can be produced by the decay of radioactive substances like uranium? Alpha, beta, pi rays Alpha, beta, gamma rays Delta, beta, gamma rays Delta, beta, plays Alpha, sigma, plays QUESTION 3 Select True or False: The proton is about 1840 times heavier than the electron. O True O False QUESTION 4 How many electrons, protons, and neutrons does an iron-55 atom have? O 26 electrons, 26 protons, and 29 neutrons 55 electrons, 26 protons, and 29 neutrons 26 electrons, 55 protons, and 29 neutrons 26 electrons, 26 protons, and 55 neutrons QUESTION 5 Almost all the mass of an atom is concentrated in the electrons O protons O nucleus O neurons O alpha particles QUESTION 6 in the nucleus of each atom of an element. The atomic number is equal to the number of O neutrons protons neutrons alpha particles O gamma rays QUESTION 7 Select True or False: The number of neutrons in all atoms of an element is the same. True False QUESTION 8 Select True or False: Isotopes are atoms of the same element that have the same atomic number but different mass numbers. True False QUESTION 9 79 Atom B 81 120 81 Atom C Atom D 80 79 118 120 80 79 The table below describes four atoms. Atom A Number of protons Number of neutrons 118 Number of electrons 79 Which atoms represent the same element? A and B represent the same element A and C represent the same element A and D represent the same element B and C represent the same element OC and D represent the same element QUESTION 10 Which, if any, defines the term molecule? A molecule represents the simplest ratio of atoms in a compound, A molecule is a unit that cannot be broken down by normal forces A molecule is an aggregate of at least two atoms in a definite arrangement held together by chemical forces. O A molecule must be composed of three atoms None of the above QUESTION 11 Give the formula for potassium oxide. OKO2 OK20 OK04 QUESTION 12 Give the formula for carbon disulfide. CSS2 O C3 4 O C2S O CS O CS2 QUESTION 13 Name the following compound: Cl207. O Chlorine oxide O Dichlorine heptoxide O Dichlorine hexoxide Dichlorine octaoxide O Dichlorine sevenoxide QUESTION 14 What do all the elements in Group I have in common? O They are all non-metals They form ions with a +1 charge They have two electrons in their outer shell They lose two electrons when forming bonds QUESTION 15 An anion is defined as a charged atom or group of atoms with a net negative charge. a a stable atom. O a group of stable atoms. an atom or group of atoms with a net positive charge. QUESTION 16 What are the two different ions present in the compound NH4NO3? NH4+, NO3+ O NH4, NO3 O N3., H7, 02- O NH43+, No4- O NH4+, NO3 QUESTION 17 Give the formula for nickel(II) sulfite. Niso O NISO3 O Ni2SO4 O Ni2(SO3) QUESTION 18 How many electrons, protons, and neutrons are in a neutral atom of the following isotope of krypton? 84 Kr O 36 electrons, 48 protons, and 36 neutrons, 84 electrons, 24 protons, and 36 neutrons 36 electrons, 36 protons, and 48 neutrons 36 electrons, 36 protons, and 84 neutrons None of the above are correct QUESTION 19 How many electrons, protons, and neutrons are in a neutral atom of the following isotope of gadolinium? 16. Gd 64 O 64 electrons, 64 protons, and 160 neutrons 64 electrons, 64 protons, and 96 neutrons O 96 electrons, 96 protons, and 64 neutrons 64 electrons, 96 protons, and 96 neutrons None of the above are correct QUESTION 20 Consider a neutral atom of the following isotope of sulfur: 34516 How many electrons, protons, and neutrons does the atom contain? O 16 electrons, 16 protons, and 18 neutrons O 18 electrons, 16 protons, and 18 neutrons O 18electrons, 16 protons, and 16 neutrons O 18electrons, 18 protons, and 18 neutrons None of the above are correct