Answered step by step
Verified Expert Solution
Question
1 Approved Answer
question 10 part A For the reaction A+B+CD+E, the initial reaction rate was measured for various initial concentrations of reactants. The following data were collected:
question 10 part A 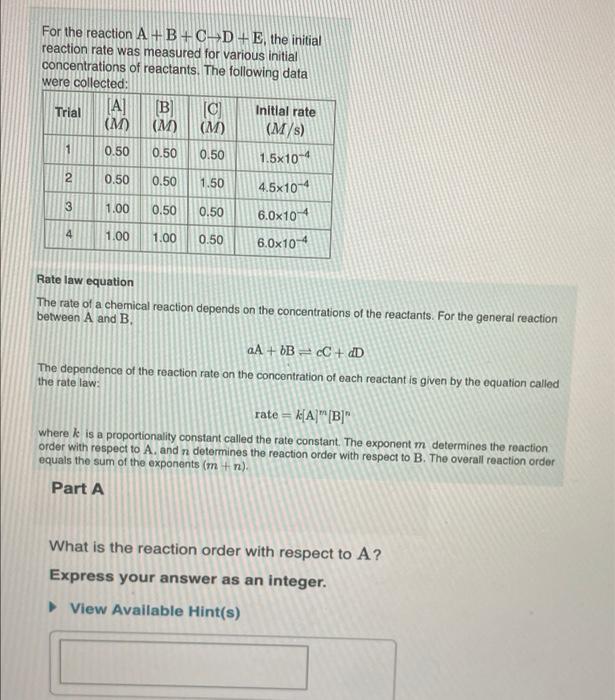
For the reaction A+B+CD+E, the initial reaction rate was measured for various initial concentrations of reactants. The following data were collected: Trial [A (B) Initial rate (M) (M) (M) (M/s) 1 1 0.50 0.50 0.50 1.5x10-4 2 0.50 0.50 1.50 4.5x10-4 3 1.00 0.50 0.50 6.0x104 4 1.00 1.00 0.50 6.0x104 Rate law equation The rate of a chemical reaction depends on the concentrations of the reactants. For the general reaction between A and B aA + bB cC + DD The dependence of the reaction rate on the concentration of each reactant is given by the equation called the rate law: rate = kA "B" where k is a proportionality constant called the rate constant. The exponent m determines the reaction order with respect to A. and n determines the reaction order with respect to B. The overall reaction order equals the sum of the exponents (m+n). Part A What is the reaction order with respect to A? Express your answer as an integer. View Available Hint(s) 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


