Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A carbon steel rigid tank initially contains a 2.5 kg of three-components gas mixture, particularly 57 kPa nitric oxide (NO), 63 kPa nitrous oxide
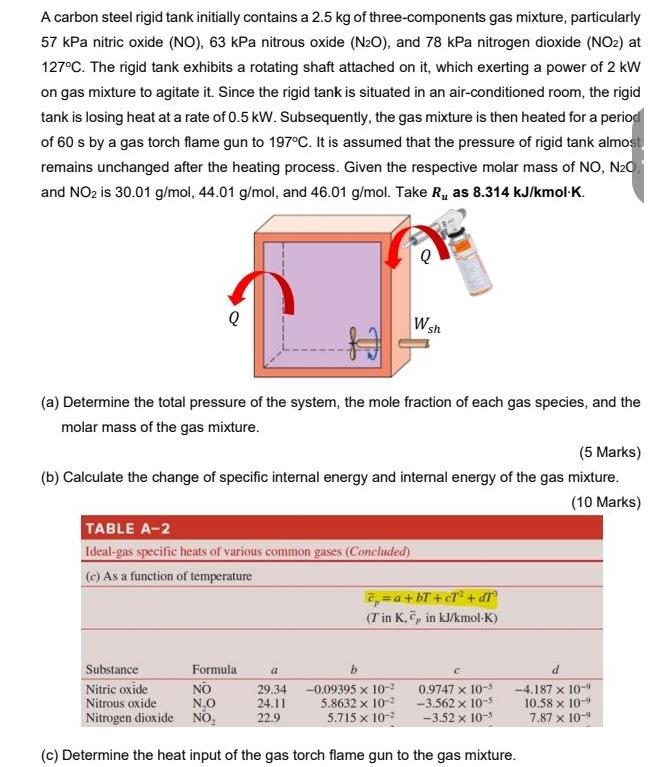
A carbon steel rigid tank initially contains a 2.5 kg of three-components gas mixture, particularly 57 kPa nitric oxide (NO), 63 kPa nitrous oxide (N2O), and 78 kPa nitrogen dioxide (NO2) at 127C. The rigid tank exhibits a rotating shaft attached on it, which exerting a power of 2 kW on gas mixture to agitate it. Since the rigid tank is situated in an air-conditioned room, the rigid tank is losing heat at a rate of 0.5 kW. Subsequently, the gas mixture is then heated for a period of 60 s by a gas torch flame gun to 197C. It is assumed that the pressure of rigid tank almost remains unchanged after the heating process. Given the respective molar mass of NO, N20 and NO2 is 30.01 g/mol, 44.01 g/mol, and 46.01 g/mol. Take R, as 8.314 kJ/kmolK. W sh (a) Determine the total pressure of the system, the mole fraction of each gas species, and the molar mass of the gas mixture. (5 Marks) (b) Calculate the change of specific internal energy and internal energy of the gas mixture. (10 Marks) TABLE A-2 Ideal-gas specific heats of various common gases (Concluded) (e) As a function of temperature 2, =a+ bT +cT* + dT (Tin K. , in kJ/kmol-K) Substance Nitric oxide Nitrous oxide Nitrogen dioxide NO, Formula NO N,0 a d -0.09395 x 10- 5.8632 x 10- 5.715 x 10-2 0.9747 x 10-5 -3.562 x 10-5 -3.52 x 10-3 -4.187 x 10- 10.58 x 10-9 7.87 x 10-9 29.34 24.11 22.9 (c) Determine the heat input of the gas torch flame gun to the gas mixture.
Step by Step Solution
★★★★★
3.43 Rating (166 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
6360fe6115fbe_235105.pdf
180 KBs PDF File
6360fe6115fbe_235105.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


