Answered step by step
Verified Expert Solution
Question
1 Approved Answer
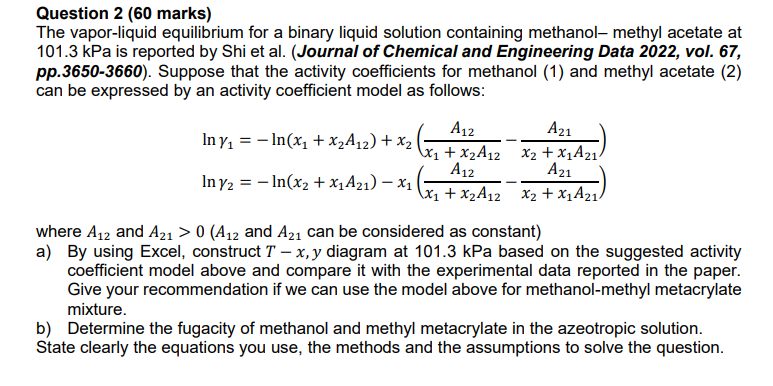
Question 2 ( 6 0 marks ) The vapor - liquid equilibrium for a binary liquid solution containing methanol - methyl acetate at 1 0
Question marks
The vaporliquid equilibrium for a binary liquid solution containing methanol methyl acetate at
kPa is reported by Shi et alJournal of Chemical and Engineering Data vol.
pp Suppose that the activity coefficients for methanol and methyl acetate
can be expressed by an activity coefficient model as follows:
where and and can be considered as constant
a By using Excel, construct diagram at kPa based on the suggested activity
coefficient model above and compare it with the experimental data reported in the paper.
Give your recommendation if we can use the model above for methanolmethyl metacrylate
mixture.
b Determine the fugacity of methanol and methyl metacrylate in the azeotropic solution.
State clearly the equations you use, the methods and the assumptions to solve the question.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


