Answered step by step
Verified Expert Solution
Question
1 Approved Answer
a) Given the following trend in proton acidity among the following hydrocarbon compounds: (N.B. the acidic proton of each compound is indicated in bold)
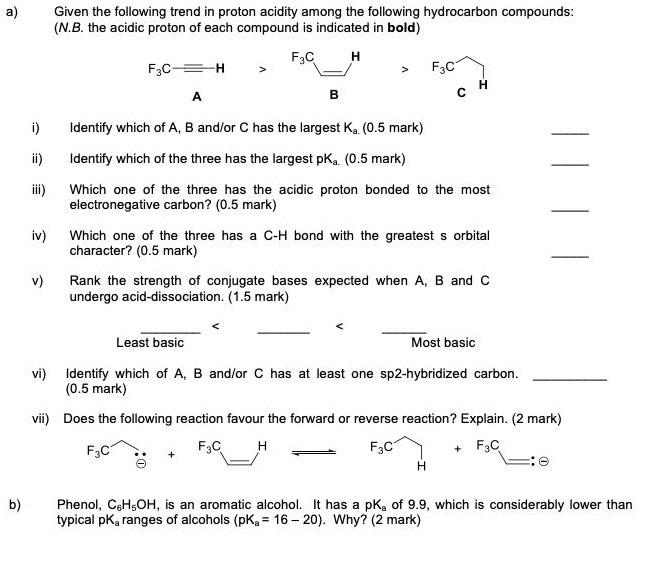
a) Given the following trend in proton acidity among the following hydrocarbon compounds: (N.B. the acidic proton of each compound is indicated in bold) F3C H F3C F3C A i) Identify which of A, B and/or C has the largest Ka. (0.5 mark) i) Identify which of the three has the largest pKa. (0.5 mark) iii) Which one of the three has the acidic proton bonded to the most electronegative carbon? (0.5 mark) iv) Which one of the three has a C-H bond with the greatest s orbital character? (0.5 mark) v) Rank the strength of conjugate bases expected when A, B and C undergo acid-dissociation. (1.5 mark) Least basic Most basic vi) Identify which of A, B and/or C has at least one sp2-hybridized carbon. (0.5 mark) vii) Does the following reaction favour the forward or reverse reaction? Explain. (2 mark) F3C F3C F3C + F3C b) Phenol, CHsOH, is an aromatic alcohol. It has a pk, of 9.9, which is considerably lower than typical pk, ranges of alcohols (pKg = 16 20). Why? (2 mark)
Step by Step Solution
★★★★★
3.39 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
B in case of phenol the phenoxide Ion produced after deprotonation is resonance stabilized th...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


