Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Question 2 (a) A rigid tank is divided into two equal parts by a partition. One part of the tank contains 2.5 kg of
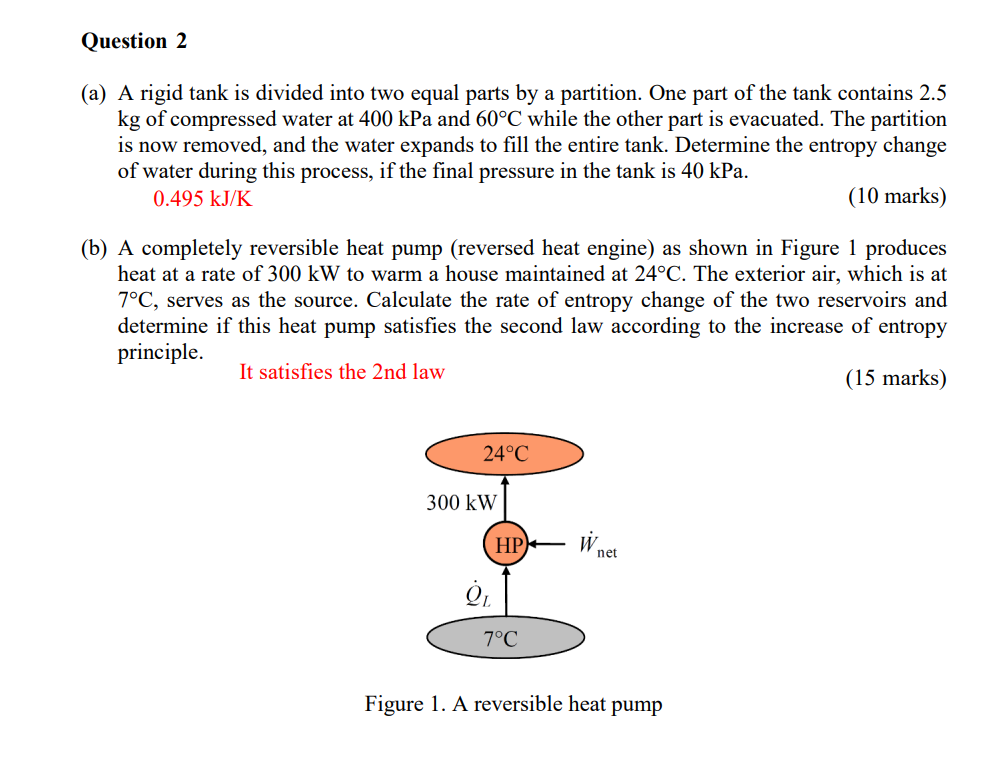
Question 2 (a) A rigid tank is divided into two equal parts by a partition. One part of the tank contains 2.5 kg of compressed water at 400 kPa and 60C while the other part is evacuated. The partition is now removed, and the water expands to fill the entire tank. Determine the entropy change of water during this process, if the final pressure in the tank is 40 kPa. 0.495 kJ/K (10 marks) (b) A completely reversible heat pump (reversed heat engine) as shown in Figure 1 produces heat at a rate of 300 kW to warm a house maintained at 24C. The exterior air, which is at 7C, serves as the source. Calculate the rate of entropy change of the two reservoirs and determine if this heat pump satisfies the second law according to the increase of entropy principle. It satisfies the 2nd law (15 marks) 24C 300 kW HP W net 7C Figure 1. A reversible heat pump
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


