Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Question 2 An air conditioner cools 225 kg of humid air/min at atmospheric pressure (1 atm), 42C and 72% relative humidity to 12C. Molecular weights
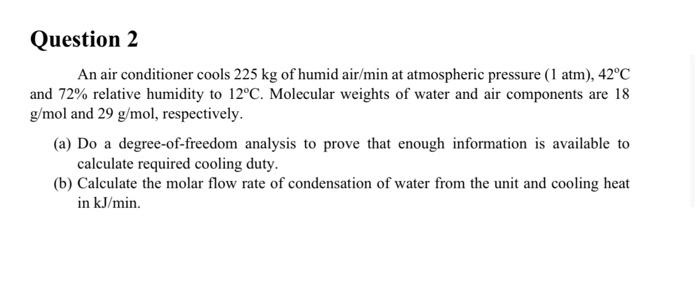
Question 2 An air conditioner cools 225 kg of humid air/min at atmospheric pressure (1 atm), 42C and 72% relative humidity to 12C. Molecular weights of water and air components are 18 g/mol and 29 g/mol, respectively. (a) Do a degree-of-freedom analysis to prove that enough information is available to calculate required cooling duty. (b) Calculate the molar flow rate of condensation of water from the unit and cooling heat in kJ/min.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


