Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Question 2 : Mark ( In the differential distillation column, a binary mixture of acetic acid and water is used, Where: The amount of the
Question :
Mark
In the differential distillation column, a binary mixture of acetic acid and water is used, Where: The amount of the liquil the container at the end of the distillation process ; Initial acetic acid concentration; Final acetic acid concentration ; The equilibrium relationship for the binary mixture of acetic acid and water is:
The amount of the liquid in the container at the start of the distillation process is:
a
b
c
Question :
Mark
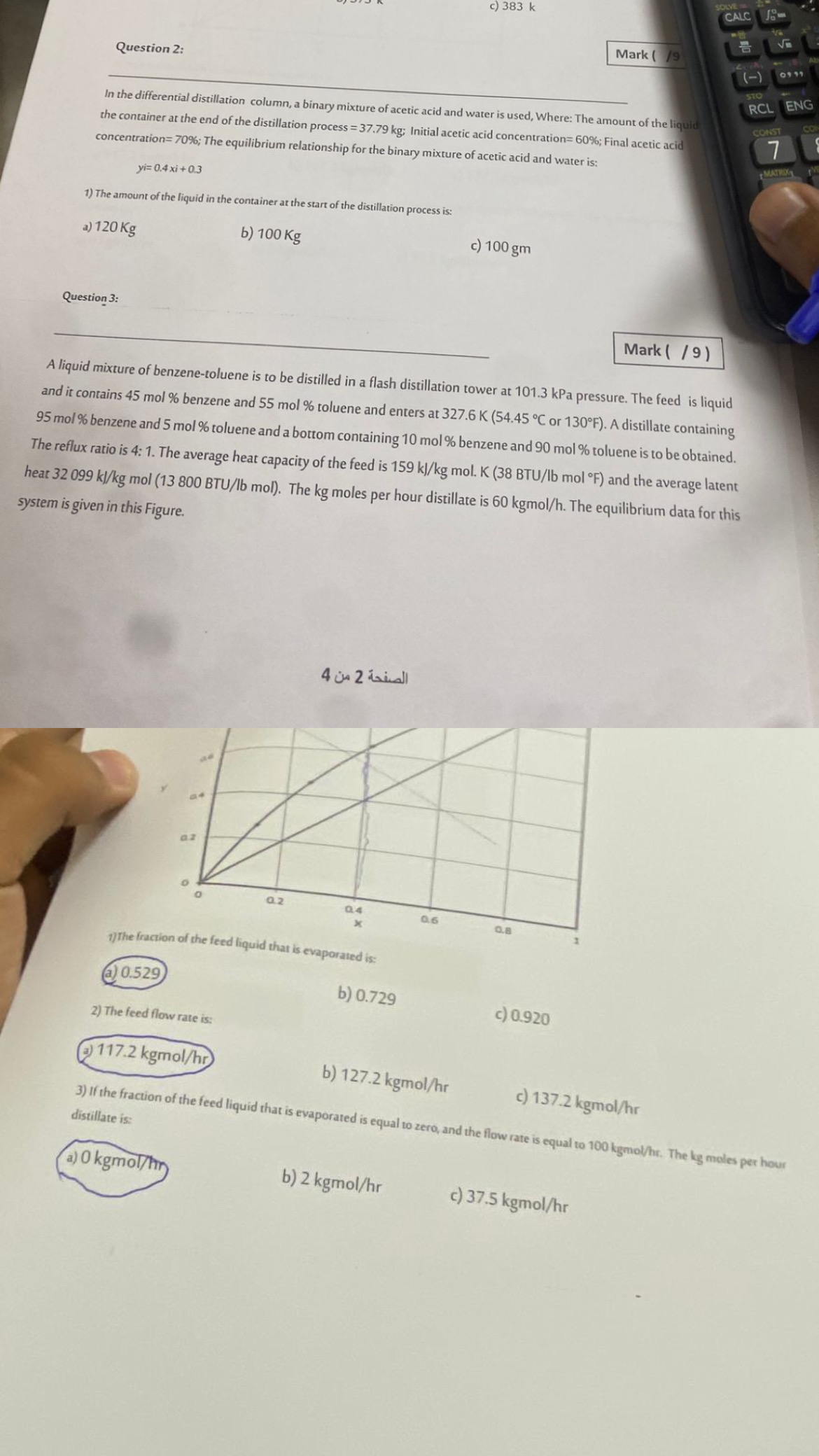
A liquid mixture of benzenetoluene is to be distilled in a flash distillation tower at kPa pressure. The feed is liquid and it contains mol benzene and mol toluene and enters at or : A distillate containing mol benzene and mol toluene and a bottom containing mol benzene and mol toluene is to be obtained. The reflux ratio is : The average heat capacity of the feed is gmol.bmol and the average latent heat gmol bmol The moles per hour distillate is kgmo The equilibrium data for this system is given in this Figure.
a
b
c
The feed flow rate is:
akgmo
bkgmo
ckgmo
If the fraction of the feed liquid that is evaporated is equal to zero, and the flow rate is equal to kgmo The moles per hour distillate is:
akgmo
bkgmo
ckgmo
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


