Answered step by step
Verified Expert Solution
Question
1 Approved Answer
question 2, please solve in aspen plus..screenshoot the answer 5.0 Tasks to be carried out Acetylene is produced from methane in the reaction: 2CH4(g)C2H2(g)+3H2(g) An
question 2, please solve in aspen plus..screenshoot the answer
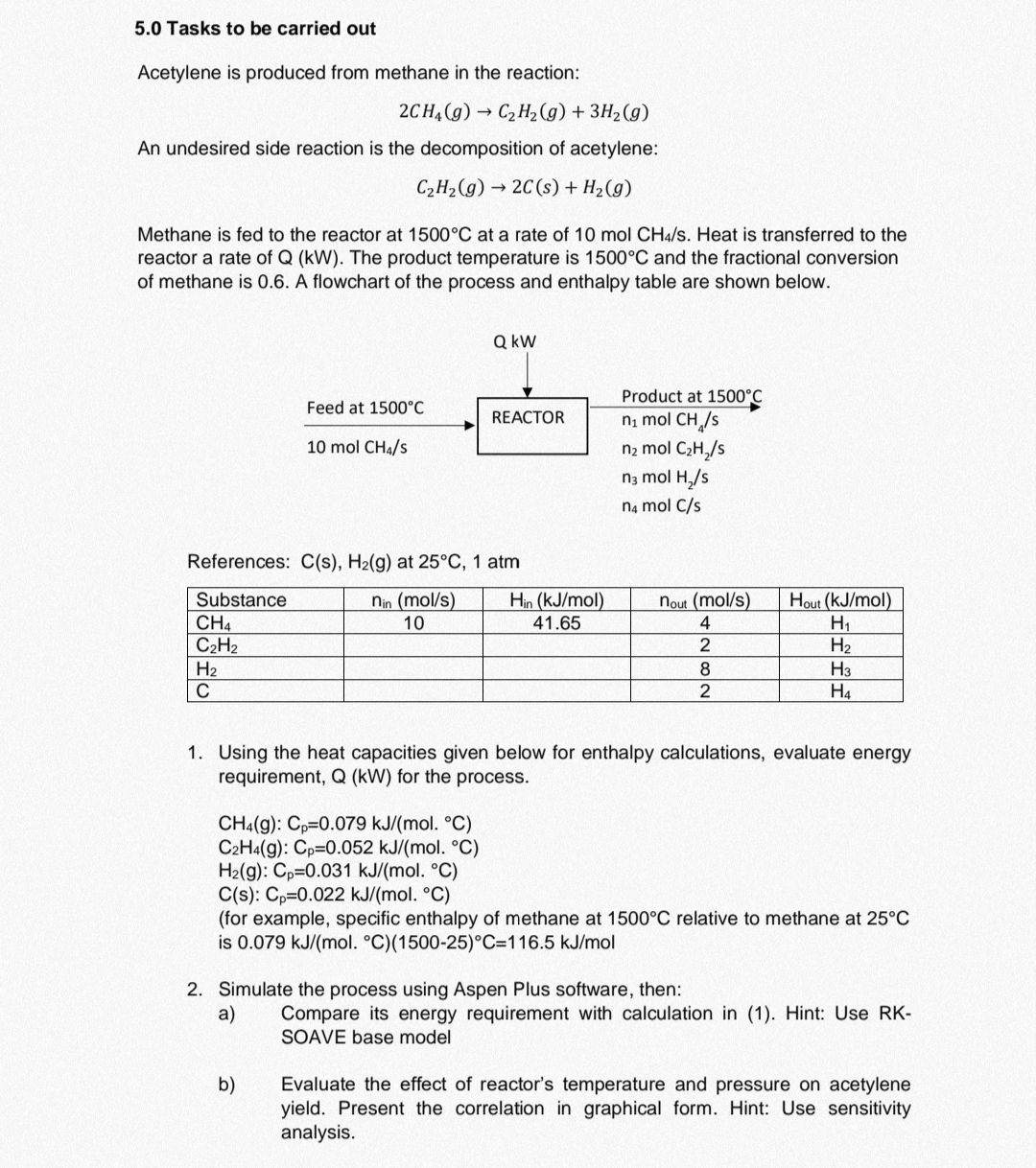 5.0 Tasks to be carried out Acetylene is produced from methane in the reaction: 2CH4(g)C2H2(g)+3H2(g) An undesired side reaction is the decomposition of acetylene: C2H2(g)2C(s)+H2(g) Methane is fed to the reactor at 1500C at a rate of 10molCH4/s. Heat is transferred to the reactor a rate of Q(kW). The product temperature is 1500C and the fractional conversion of methane is 0.6 . A flowchart of the process and enthalpy table are shown below. References: C(s),H2(g) at 25C, 1 atm 1. Using the heat capacities given below for enthalpy calculations, evaluate energy requirement, Q(kW) for the process. CH4(g):Cp=0.079kJ/(molC)C2H4(g):Cp=0.052kJ/(mol.C)H2(g):Cp=0.031kJ/(mol.C)C(s):Cp=0.022kJ/(mol.C) (for example, specific enthalpy of methane at 1500C relative to methane at 25C is 0.079kJ/(mol.C)(150025)C=116.5kJ/mol 2. Simulate the process using Aspen Plus software, then: a) Compare its energy requirement with calculation in (1). Hint: Use RKSOAVE base model b) Evaluate the effect of reactor's temperature and pressure on acetylene yield. Present the correlation in graphical form. Hint: Use sensitivity analysis
5.0 Tasks to be carried out Acetylene is produced from methane in the reaction: 2CH4(g)C2H2(g)+3H2(g) An undesired side reaction is the decomposition of acetylene: C2H2(g)2C(s)+H2(g) Methane is fed to the reactor at 1500C at a rate of 10molCH4/s. Heat is transferred to the reactor a rate of Q(kW). The product temperature is 1500C and the fractional conversion of methane is 0.6 . A flowchart of the process and enthalpy table are shown below. References: C(s),H2(g) at 25C, 1 atm 1. Using the heat capacities given below for enthalpy calculations, evaluate energy requirement, Q(kW) for the process. CH4(g):Cp=0.079kJ/(molC)C2H4(g):Cp=0.052kJ/(mol.C)H2(g):Cp=0.031kJ/(mol.C)C(s):Cp=0.022kJ/(mol.C) (for example, specific enthalpy of methane at 1500C relative to methane at 25C is 0.079kJ/(mol.C)(150025)C=116.5kJ/mol 2. Simulate the process using Aspen Plus software, then: a) Compare its energy requirement with calculation in (1). Hint: Use RKSOAVE base model b) Evaluate the effect of reactor's temperature and pressure on acetylene yield. Present the correlation in graphical form. Hint: Use sensitivity analysis Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


