Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Question 22 Not yet answered Marked out of 1.00 P Flag question NaOH reacts with H2SO4 as follows: 2NaOH + H2SO4 Na2SO4 + 2H20 Calculate
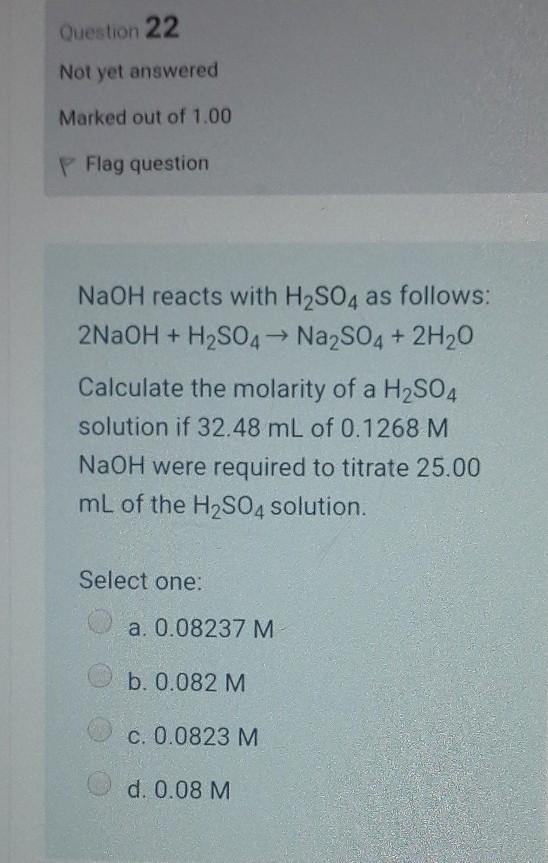
Question 22 Not yet answered Marked out of 1.00 P Flag question NaOH reacts with H2SO4 as follows: 2NaOH + H2SO4 Na2SO4 + 2H20 Calculate the molarity of a H2SO4 solution if 32.48 mL of 0.1268 M NaOH were required to titrate 25.00 mL of the H2SO4 solution. Select one: a. 0.08237 M b. 0.082 M C. 0.0823 M d. 0.08 M
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


