Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Question 3 1 pts A metal gas cylinder is at a pressure of 106.7 arm and contain 16.7 moles of chlorine gas (Cl2). It
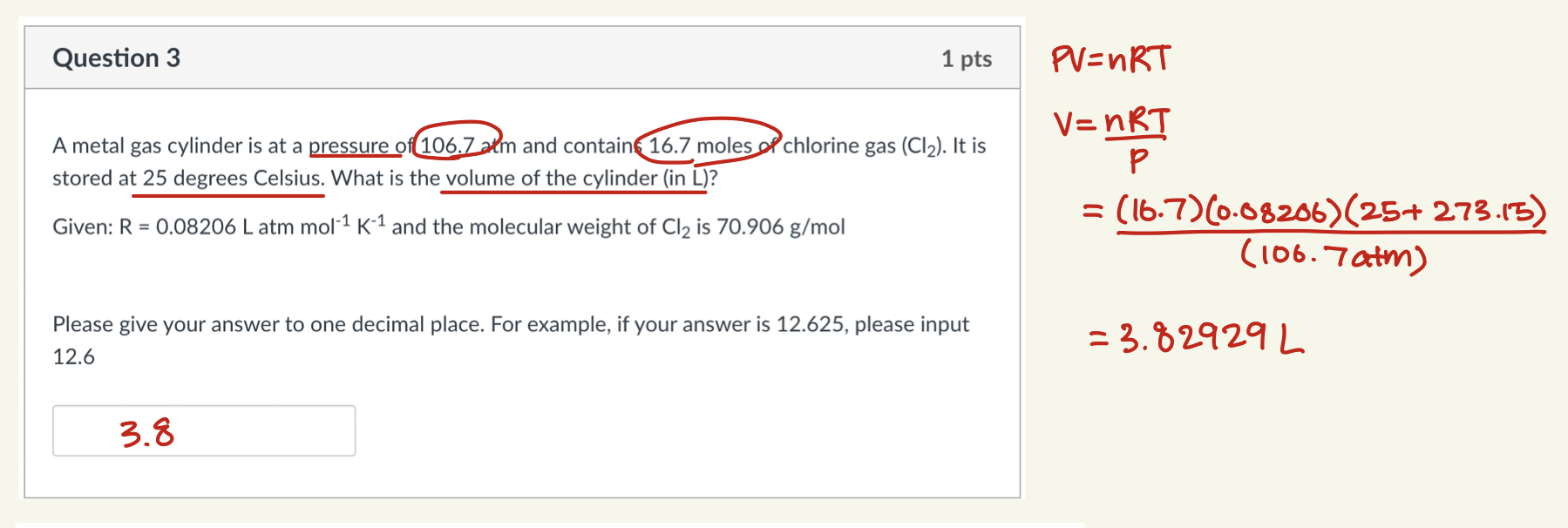
Question 3 1 pts A metal gas cylinder is at a pressure of 106.7 arm and contain 16.7 moles of chlorine gas (Cl2). It is stored at 25 degrees Celsius. What is the volume of the cylinder (in L)? Given: R = 0.08206 L atm mol-1 K-1 and the molecular weight of Cl2 is 70.906 g/mol Please give your answer to one decimal place. For example, if your answer is 12.625, please input 12.6 PV=nRT V = nRT P = (16.7) (0.08206) (25+273.15) (106.7atm) =3.829292 3.8
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


