Answered step by step
Verified Expert Solution
Question
1 Approved Answer
For a binary system consist of ortho-xylene (o-xylene) [species 1] and para-xylene (p-xylene) [species 2], this system is found to obey Raoult's law. It
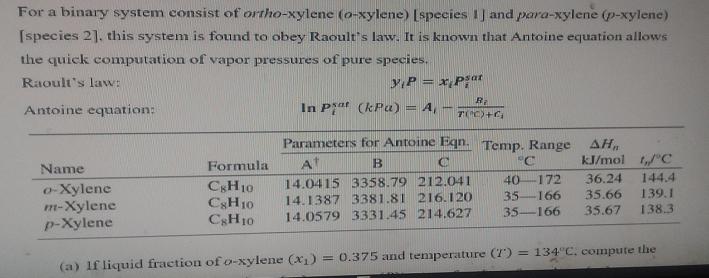


For a binary system consist of ortho-xylene (o-xylene) [species 1] and para-xylene (p-xylene) [species 2], this system is found to obey Raoult's law. It is known that Antoine equation allows the quick computation of vapor pressures of pure species. Raoult's law: yP = xpsat Antoine equation: Parameters for Antoine Eqn. Temp. Range , At B C C 14.0415 3358.79 212.041 14.1387 3381.81 216.120 14.0579 3331.45 214.627 40 172 35-166 35-166 kJ/mol t,/C 36.24 144,4 35.66 139.1 35.67 138.3 (a) If liquid fraction of o-xylene (x) = 0.375 and temperature (7) = 134C, compute the Name o-Xylene m-Xylene p-Xylene R. In Pat (kPa) = A7(C)+ Formula C8H10 C8H10 C8H10 m-Xylene p-Xylene B10 CgH10 C8H10 14.04 40 -172 36.24 144.4 3358.79 212.041 14.1387 3381.81 216.120 14.0579 3331.45 214.627 35-166 35.67 138.3 35-166 35.66 139.1 (a) If liquid fraction of o-xylene (x) = 0.375 and temperature (7) = 134C, compute the liquid fraction of p-xylene (x). pressure (P), vapor fraction of o-xylene (v), vapor fraction of p-xylene (y), vapor pressure of o-xylene (P), and vapor pressure of p xylene (P)- (8 marks) (b) If vapor fraction of p-xylene (y2) = 0.458 and temperature (7) = 162C, compute the vapor fraction of o-xylene (y). pressure (P), liquid fraction of o-xylene (x), and liquid fraction of p-xylene (x). vapor fraction of o-xylene (y), pressure (P), liquid fraction of o-xylene (x), and liquid fraction of p-xylene (x). (6 marks) (c) If liquid fraction of p-xylene (x) = 0.771 and pressure (P) = 95.27 kPa, calculate the liquid fraction of o-xylene (x) and temperature (T). END OF QUESTION PAPER (6 Marks)
Step by Step Solution
★★★★★
3.40 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
a Minture have orth Xylen 0 Pona Xylene P X0375 T 154C Since it Bimay to Minture i...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


