Answered step by step
Verified Expert Solution
Question
1 Approved Answer
question 3, 4, 5, & 6 using the data given. trials 1,2 & 3 were room temp and trial 4 was in hot water bath
question 3, 4, 5, & 6 using the data given. 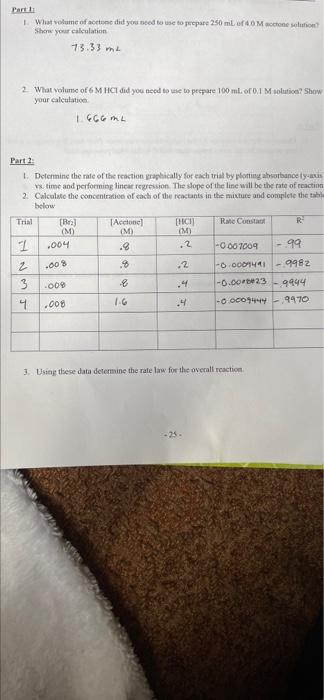



trials 1,2 & 3 were room temp and trial 4 was in hot water bath of 45C
Parti What volume of action did you need to use to prepare 250 ml of Mone solution Show your calculation 73.33 m 2 What volume of 6 M HCI did you need to use to prepare 100 ml. of 0,1 M solutical Show your calculation 1666 Part 2 1. Determine the rate of the reaction graphically for each trial by plotting absorbance lys s time and performing linear regression. The slope of the line will be the rate of reaction 2 Calculate the concentration of each of the reactants in the mixture and complete the table below Trial [B Acetone) [HACIJ Rahe Const R M M (M I ,004 .8 .2 -0.007009 -99 2 % 2 -O DOO -9982 3 009 8 .4 -0.00-23 9444 IG 4 -0.00074449470 .00% .00 3. Using these data determine the ralelow for the overall reaction -25. Chemistry Lab CHEM 112 4 Determine the rate constant for each room temperature experiment is the rate constant truly constant? What do you observe about the rate constant Part 5. Determine the rate constant for the higher temperature trials. How does this compare to the rate constant for the room temperature trial is this what you expected 6. Using the rate constants from the room temperature trials and heated trials determine the activation energy for this reaction Parti What volume of action did you need to use to prepare 250 ml of Mone solution Show your calculation 73.33 m 2 What volume of 6 M HCI did you need to use to prepare 100 ml. of 0,1 M solutical Show your calculation 1666 Part 2 1. Determine the rate of the reaction graphically for each trial by plotting absorbance lys s time and performing linear regression. The slope of the line will be the rate of reaction 2 Calculate the concentration of each of the reactants in the mixture and complete the table below Trial [B Acetone) [HACIJ Rahe Const R M M (M I ,004 .8 .2 -0.007009 -99 2 % 2 -O DOO -9982 3 009 8 .4 -0.00-23 9444 IG 4 -0.00074449470 .00% .00 3. Using these data determine the ralelow for the overall reaction -25. Chemistry Lab CHEM 112 4 Determine the rate constant for each room temperature experiment is the rate constant truly constant? What do you observe about the rate constant Part 5. Determine the rate constant for the higher temperature trials. How does this compare to the rate constant for the room temperature trial is this what you expected 6. Using the rate constants from the room temperature trials and heated trials determine the activation energy for this reaction Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


