Answered step by step
Verified Expert Solution
Question
1 Approved Answer
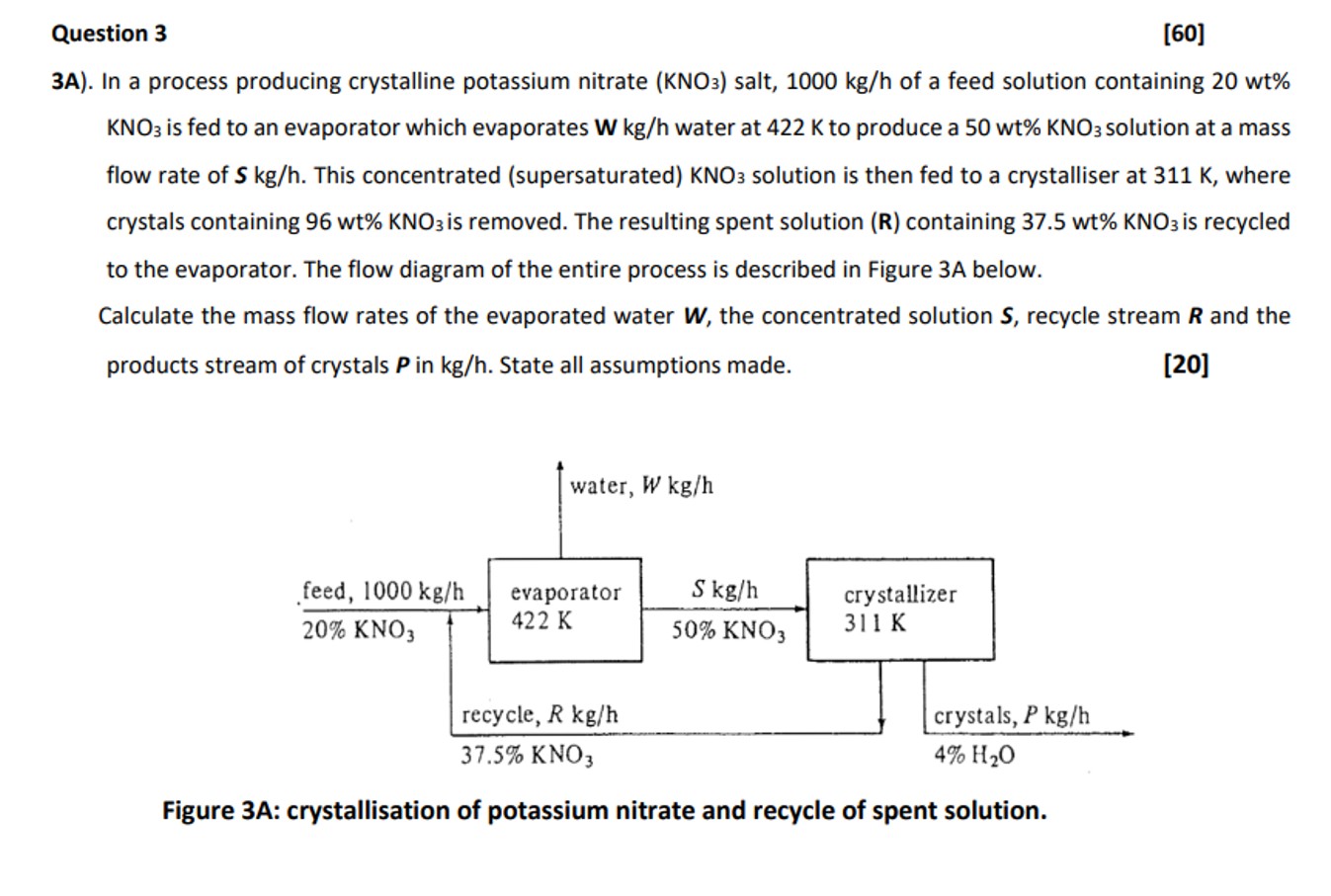
Question 3 [ 6 0 ] 3 A ) . In a process producing crystalline potassium nitrate ( K N O 3 ) salt, 1
Question
A In a process producing crystalline potassium nitrate salt, of a feed solution containing is fed to an evaporator which evaporates water at to produce a solution at a mass flow rate of This concentrated supersaturated solution is then fed to a crystalliser at where crystals containing is removed. The resulting spent solution containing is recycled to the evaporator. The flow diagram of the entire process is described in Figure A below.
Calculate the mass flow rates of the evaporated water the concentrated solution recycle stream and the products stream of crystals in State all assumptions made.
Figure sA: crystailsation or potassum ntrate ana recyce or spent soluton
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


