Answered step by step
Verified Expert Solution
Question
1 Approved Answer
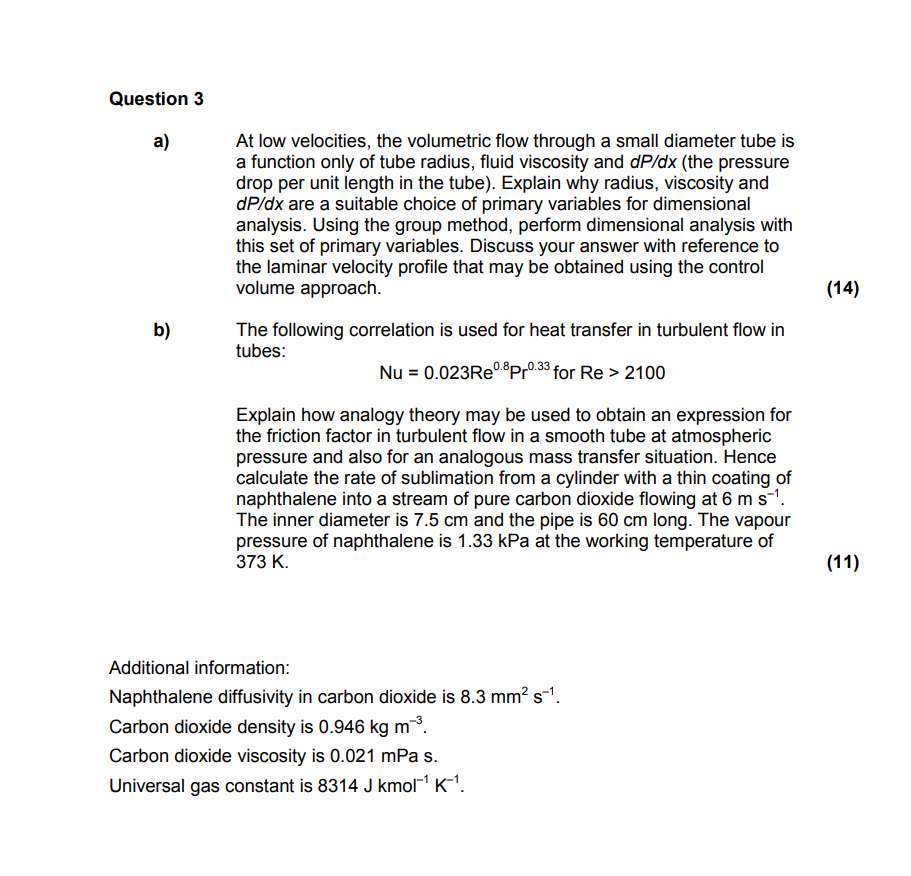
Question 3 a ) At low velocities, the volumetric flow through a small diameter tube is a function only of tube radius, fluid viscosity and
Question
a At low velocities, the volumetric flow through a small diameter tube is
a function only of tube radius, fluid viscosity and the pressure
drop per unit length in the tube Explain why radius, viscosity and
are a suitable choice of primary variables for dimensional
analysis. Using the group method, perform dimensional analysis with
this set of primary variables. Discuss your answer with reference to
the laminar velocity profile that may be obtained using the control
volume approach.
b The following correlation is used for heat transfer in turbulent flow in
tubes:
for
Explain how analogy theory may be used to obtain an expression for
the friction factor in turbulent flow in a smooth tube at atmospheric
pressure and also for an analogous mass transfer situation. Hence
calculate the rate of sublimation from a cylinder with a thin coating of
naphthalene into a stream of pure carbon dioxide flowing at
The inner diameter is and the pipe is long. The vapour
pressure of naphthalene is kPa at the working temperature of
Additional information:
Naphthalene diffusivity in carbon dioxide is
Carbon dioxide density is
Carbon dioxide viscosity is mPas.
Universal gas constant is
Use the correlation to find specific values and no assumptions to be made using the correlations.
show step by step calucaltions to receive marks for both questions.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


