Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Question 3 Consider the PEM fuel cell. What is the power that can be transferred to a load if the cell provides 0.38V(VL) at 10000A
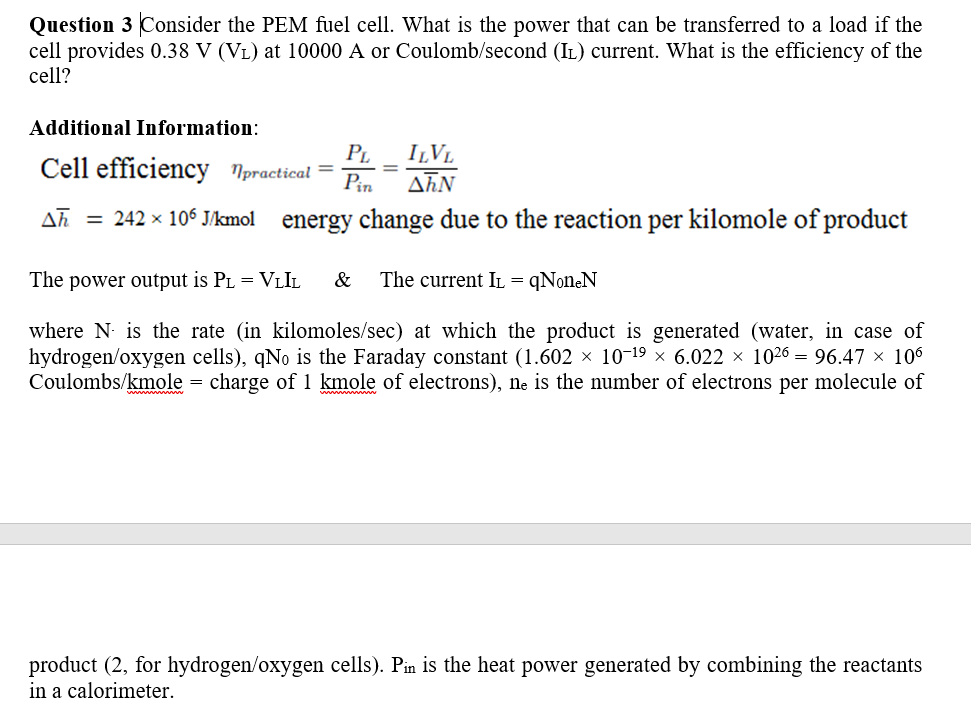 Question 3 Consider the PEM fuel cell. What is the power that can be transferred to a load if the cell provides 0.38V(VL) at 10000A or Coulomb/second ( IL) current. What is the efficiency of the cell? Additional Information: Cell efficiency practical=PinPL=hNILVL h=242106J/kmol energy change due to the reaction per kilomole of product The power output is PL=VLIL& \& current IL=qNneN where N. is the rate (in kilomoles/sec) at which the product is generated (water, in case of hydrogen/oxygen cells), qN0 is the Faraday constant (1.60210196.0221026=96.47106 Coulombs /kmole= charge of 1kmole of electrons), ne is the number of electrons per molecule of product ( 2 , for hydrogen/oxygen cells). Pin is the heat power generated by combining the reactants in a calorimeter
Question 3 Consider the PEM fuel cell. What is the power that can be transferred to a load if the cell provides 0.38V(VL) at 10000A or Coulomb/second ( IL) current. What is the efficiency of the cell? Additional Information: Cell efficiency practical=PinPL=hNILVL h=242106J/kmol energy change due to the reaction per kilomole of product The power output is PL=VLIL& \& current IL=qNneN where N. is the rate (in kilomoles/sec) at which the product is generated (water, in case of hydrogen/oxygen cells), qN0 is the Faraday constant (1.60210196.0221026=96.47106 Coulombs /kmole= charge of 1kmole of electrons), ne is the number of electrons per molecule of product ( 2 , for hydrogen/oxygen cells). Pin is the heat power generated by combining the reactants in a calorimeter Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


