Answered step by step
Verified Expert Solution
Question
1 Approved Answer
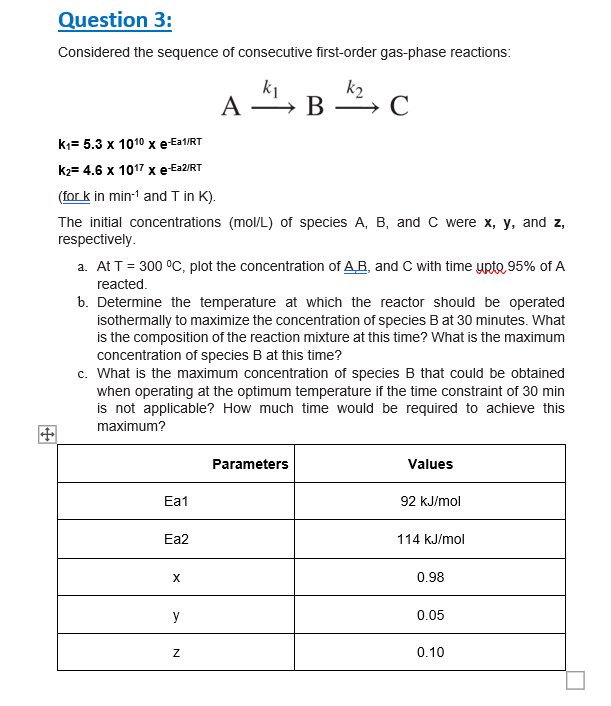
Question 3 : Considered the sequence of consecutive first - order gas - phase reactions: A k 1 B k 2 C k 1 =
Question :
Considered the sequence of consecutive firstorder gasphase reactions:
fork in min and in
The initial concentrations molL of species and were and
respectively.
a At plot the concentration of and with time upte of
reacted.
b Determine the temperature at which the reactor should be operated
isothermally to maximize the concentration of species at minutes. What
is the composition of the reaction mixture at this time? What is the maximum
concentration of species at this time?
c What is the maximum concentration of species that could be obtained
when operating at the optimum temperature if the time constraint of min
is not applicable? How much time would be required to achieve this
maximum?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


