Question
Apply the Gibbs phase rule to determine the number of independent primary reactions needed to calculate the equilibrium composition of the butadiene system at
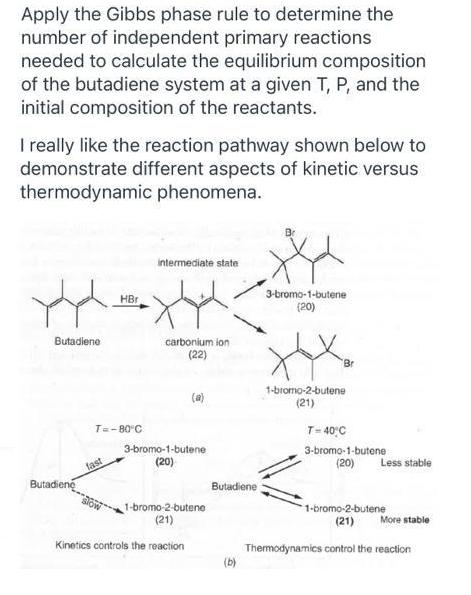
Apply the Gibbs phase rule to determine the number of independent primary reactions needed to calculate the equilibrium composition of the butadiene system at a given T, P, and the initial composition of the reactants. I really like the reaction pathway shown below to demonstrate different aspects of kinetic versus thermodynamic phenomena. intermediate state 3-bromo-1-butene (20) HBr Butadiene carbonium ion (22) Br 1-bromo-2-butene (21) (a) T=-80 C T- 40C 3-bromo-1-butene 3-bromo-1-butene (20) fast (20) Less stable Butadiene Butadiene 1-bromo-2-butene (21) 1-bromo-2-butene (21) More stable Kinetics controls the reaction Thermodynamics control the reaction (b)
Step by Step Solution
3.48 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
General Chemistry
Authors: Darrell Ebbing, Steven D. Gammon
9th edition
978-0618857487, 618857486, 143904399X , 978-1439043998
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



