Question
[Question 3] Is entropy change of these processes positive or negative or constant? 8 points (a) 2H2 + O2 = 2HO (b) 302+4Fe=2Fe2O3 (c)
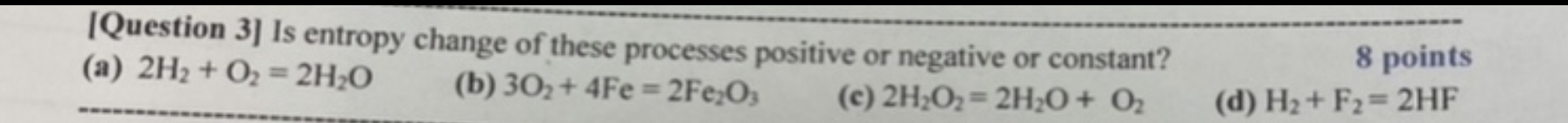
[Question 3] Is entropy change of these processes positive or negative or constant? 8 points (a) 2H2 + O2 = 2HO (b) 302+4Fe=2Fe2O3 (c) 2H2O2=2HO+ O (d) H+ F2=2HF
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Modern Quantum Mechanics
Authors: J.J Sakurai
Revised Edition
9781108499996, 201539292, 978-0201539295
Students also viewed these Mechanical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



