Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Question 3 Not yet answered Marked out of 1.00 Flag question The interface between blood and stainless steel makes an angle of 110. What would
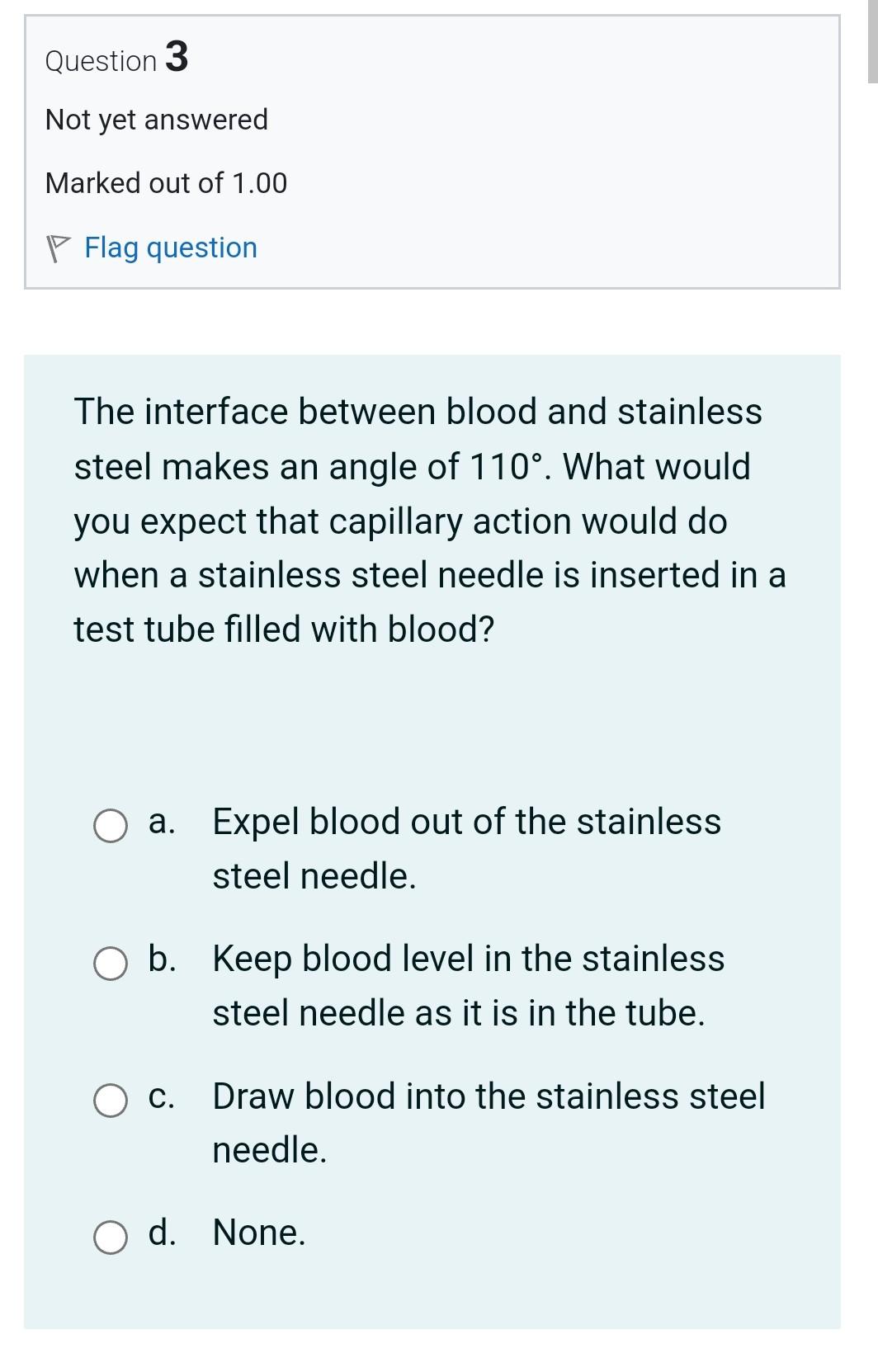
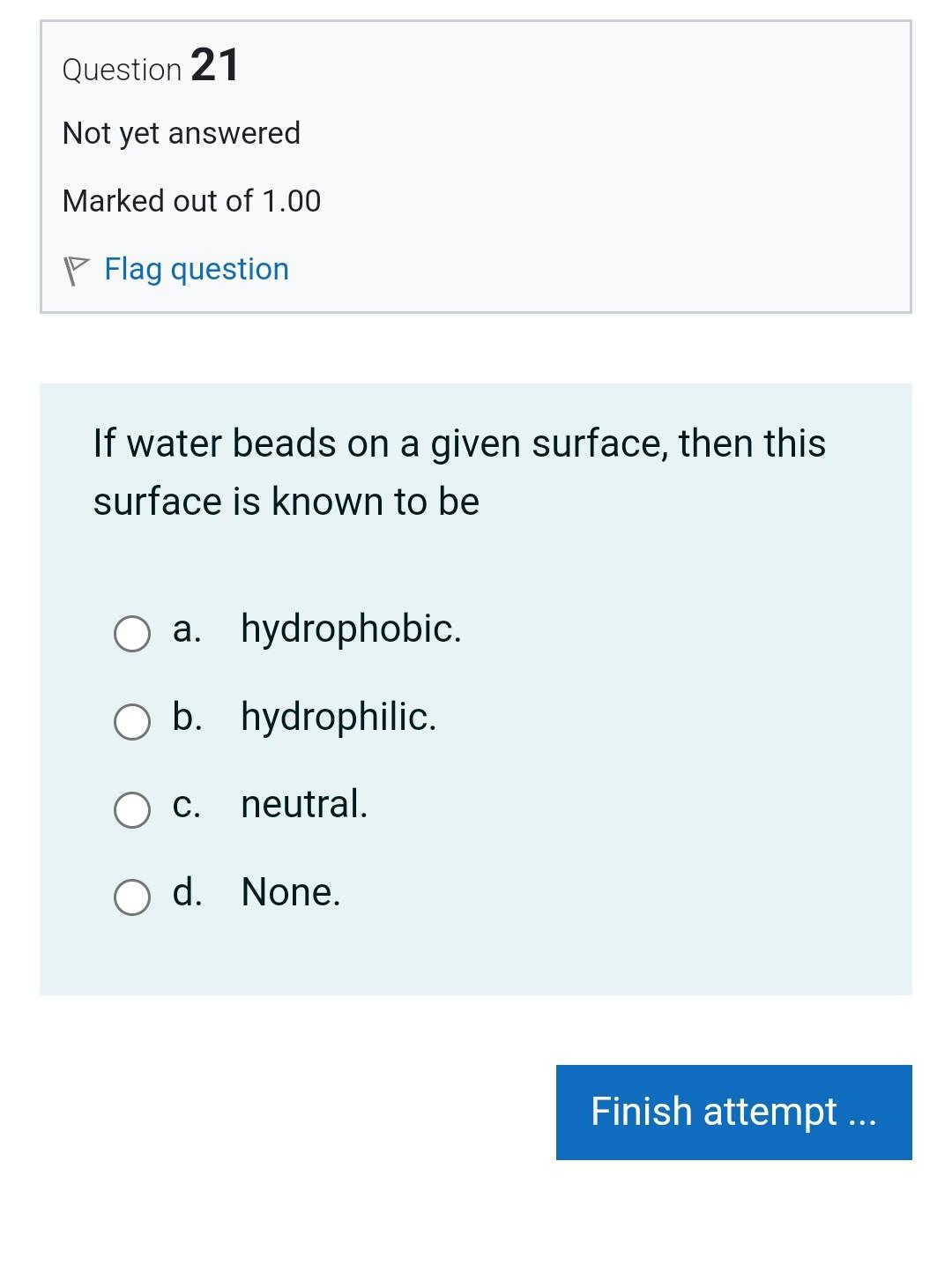
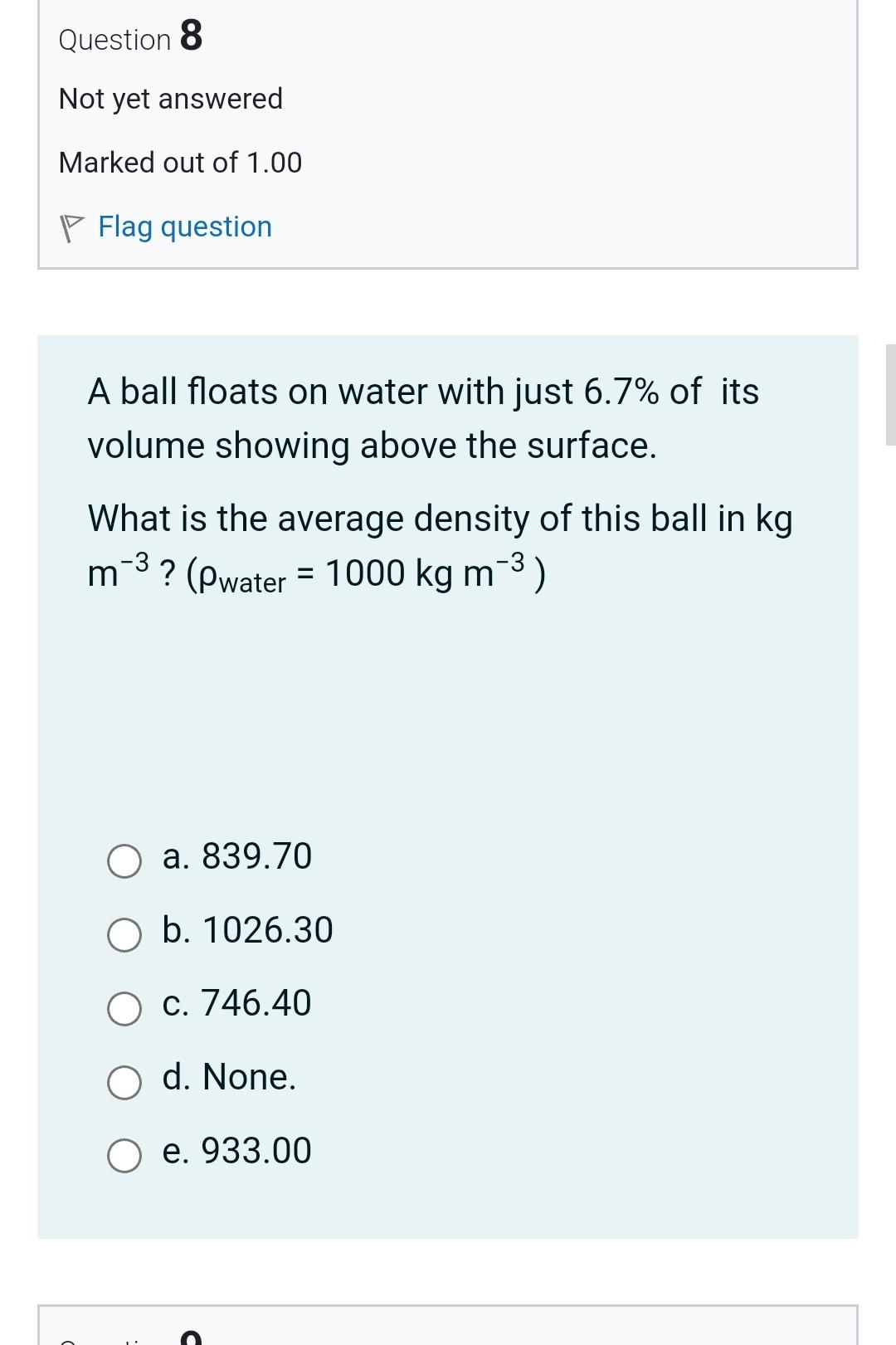






Question 3 Not yet answered Marked out of 1.00 Flag question The interface between blood and stainless steel makes an angle of 110. What would you expect that capillary action would do when a stainless steel needle is inserted in a test tube filled with blood? a. Expel blood out of the stainless steel needle. b. Keep blood level in the stainless steel needle as it is in the tube. c. Draw blood into the stainless steel needle. d. None. Question 21 Not yet answered Marked out of 1.00 Flag question If water beads on a given surface, then this surface is known to be a. hydrophobic. b. hydrophilic. c. neutral. d. None. Question 8 Not yet answered Marked out of 1.00 Flag question A ball floats on water with just 6.7% of its volume showing above the surface. What is the average density of this ball in kg m3 ? (water=1000kgm3) a. 839.70 b. 1026.30 c. 746.40 d. None. e. 933.00 Question 4 Not yet answered Marked out of 1.00 Flag question In the human lung, alveoli of different sizes are stable, due to a. None. b. the presence of oxygen. c. the presence of pulmonary surfactant. d. the presence of carbon dioxide. e. the presence of nitrogen. Question 5 Not yet answered Question 15 Not yet answered Marked out of 1.00 Flag question A student standardizes the concentration of a salt-water solution by slowly adding salt until an egg will just float. The procedure is based on the assumption that: a. all eggs have the same density b. all eggs have the same weight c. all eggs have the same volume d. the salt tends to neutralize the cholesterol in the egg e. all eggs have the same shape Question 16 Not yet answered Marked out of 1.00 Question 7 Not yet answered Marked out of 1.00 Flag question A wooden cube 5cm on a side floats level on water with just 8.5mm of the cube immersed below the surface. What is the density of the wood in kgm3 ? ( water=1000kg m3 ) a. None. b. 187.00 c. 170.00 d. 153.00 e. 136.00 Question 20 Not yet answered Marked out of 1.00 Flag question Let Fc be the cohesive forces between water molecules and Fa be the adhesive forces between water molecules and molecules of the surface. If water beads on a surface, then a. Cannot judge because the surface is unknown. b. Fc=Fa c. Fc
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


