Answered step by step
Verified Expert Solution
Question
1 Approved Answer
QUESTION 3 The below set-up is a double-fluid manometer that consists of the liquid ammonia (NH3) and the unknown gauge fluid. The manometer is
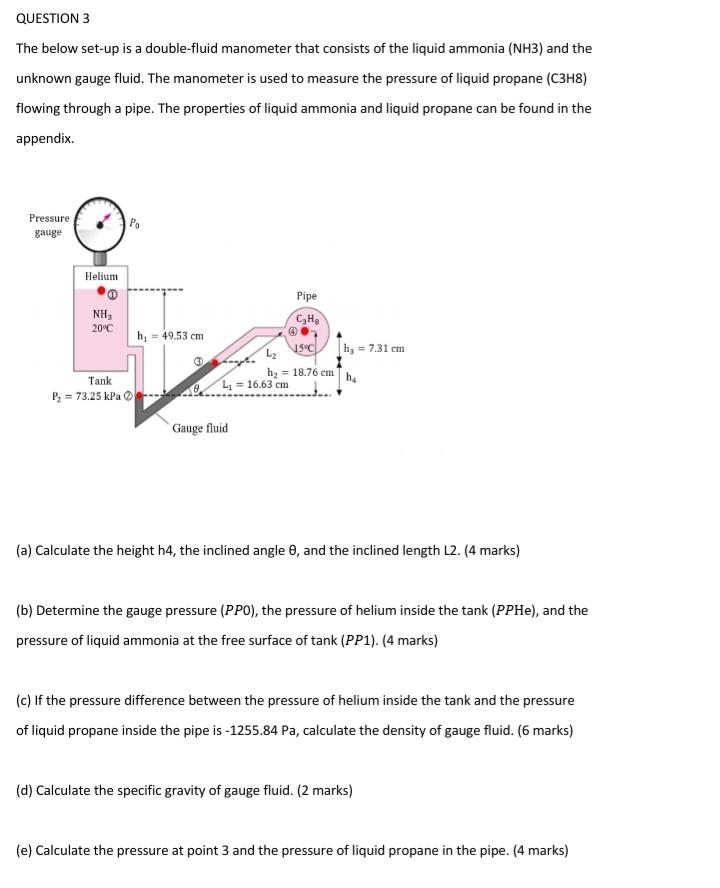
QUESTION 3 The below set-up is a double-fluid manometer that consists of the liquid ammonia (NH3) and the unknown gauge fluid. The manometer is used to measure the pressure of liquid propane (C3H8) flowing through a pipe. The properties of liquid ammonia and liquid propane can be found in the appendix. Pressure gauge Helium NH3 20C Tank P= 73.25 kPa h = 49.53 cm L = 16.63 cm Gauge fluid Pipe CH 4 15C L h= 18.76 cm |h = 7.31 cm h (a) Calculate the height h4, the inclined angle 0, and the inclined length L2. (4 marks) (b) Determine the gauge pressure (PPO), the pressure of helium inside the tank (PPHe), and the pressure of liquid ammonia at the free surface of tank (PP1). (4 marks) (c) If the pressure difference between the pressure of helium inside the tank and the pressure of liquid propane inside the pipe is -1255.84 Pa, calculate the density of gauge fluid. (6 marks) (d) Calculate the specific gravity of gauge fluid. (2 marks) (e) Calculate the pressure at point 3 and the pressure of liquid propane in the pipe. (4 marks)
Step by Step Solution
★★★★★
3.49 Rating (162 Votes )
There are 3 Steps involved in it
Step: 1
Question 3 2 we calculate the height hy th...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


