Answered step by step
Verified Expert Solution
Question
1 Approved Answer
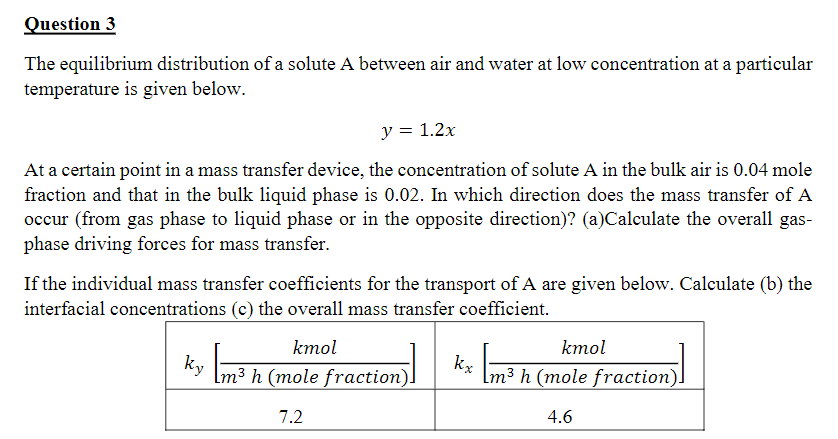
Question 3 The equilibrium distribution of a solute A between air and water at low concentration at a particular temperature is given below. y =
Question
The equilibrium distribution of a solute A between air and water at low concentration at a particular temperature is given below.
At a certain point in a mass transfer device, the concentration of solute in the bulk air is mole fraction and that in the bulk liquid phase is In which direction does the mass transfer of A occur from gas phase to liquid phase or in the opposite direction
aCalculate the overall gasphase driving forces for mass transfer.
If the individual mass transfer coefficients for the transport of A are given below. Calculate
b the interfacial concentrations c the overall mass transfer coefficient.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
To solve this problem lets tackle each part step by step Part a Determining the Direction of Mass Transfer and Overall GasPhase Driving Forces 1 Given ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


