Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Question 4 (15 points) Please report the following for TRIAL 2 (you do not need to write a verbal description of the calculations since you

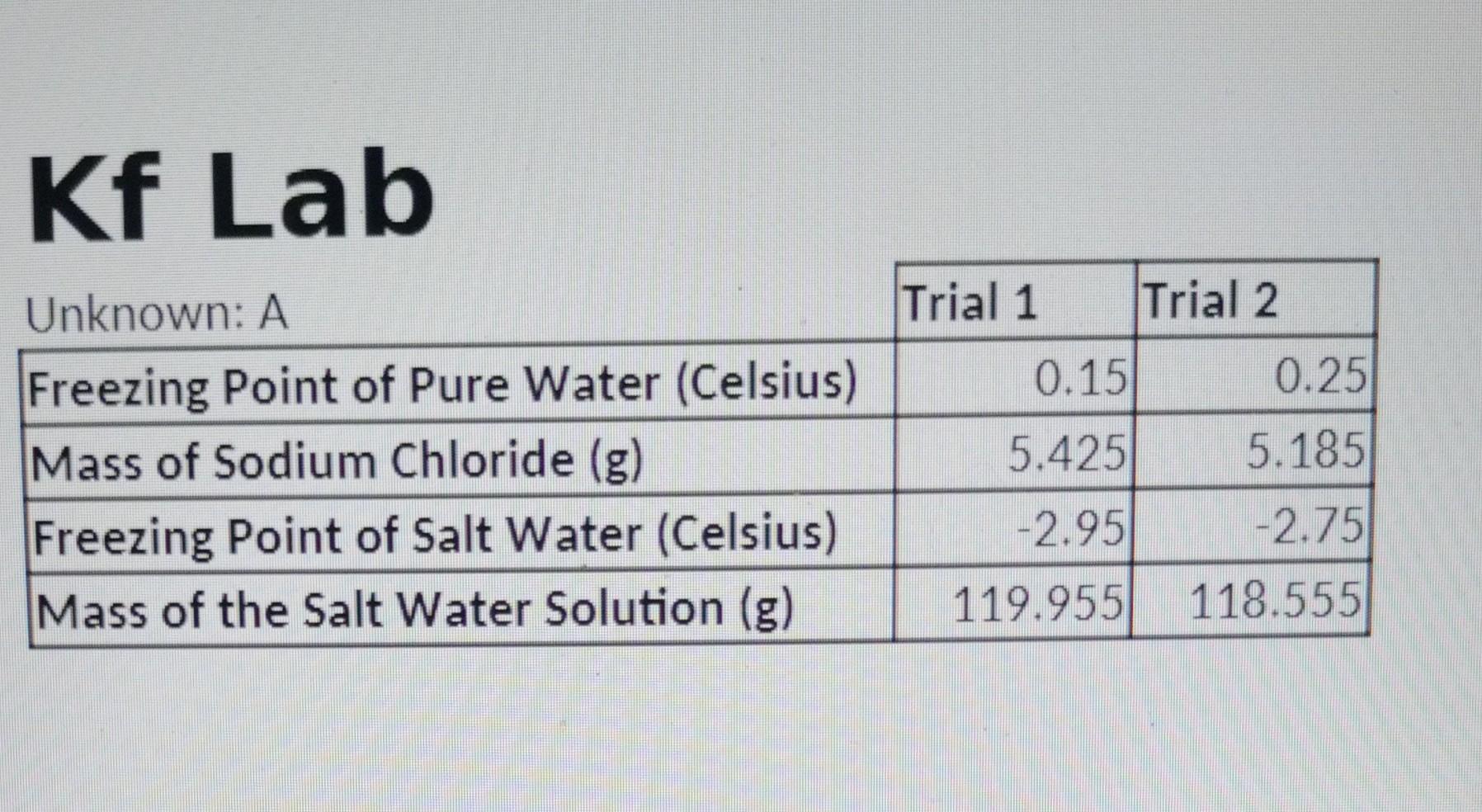
Question 4 (15 points) Please report the following for TRIAL 2 (you do not need to write a verbal description of the calculations since you already did that for trial 1 in the previous answers): a) molality of the NaCl solution b) freezing point depression (change in T ) c) freezing point depression constant (Kf) d) Percent Error of the Kf Kf Lab \begin{tabular}{l|r|r|} \cline { 2 - 3 } \multicolumn{1}{l|}{ Unknown: A } & \multicolumn{1}{|c|}{ Trial 1 } & \multicolumn{1}{l|}{ Trial 2 } \\ \hline Freezing Point of Pure Water (Celsius) & 0.15 & 0.25 \\ \hline Mass of Sodium Chloride (g) & 5.425 & 5.185 \\ \hline Freezing Point of Salt Water (Celsius) & 2.95 & 2.75 \\ \hline Mass of the Salt Water Solution (g) & 119.955 & 118.555 \\ \hline \end{tabular}
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


