Question
Question 4: A system consisting of 1kg H0 undergoes the below processes: Process 1-2: Constant volume heating from P = 500 kPa, T =160
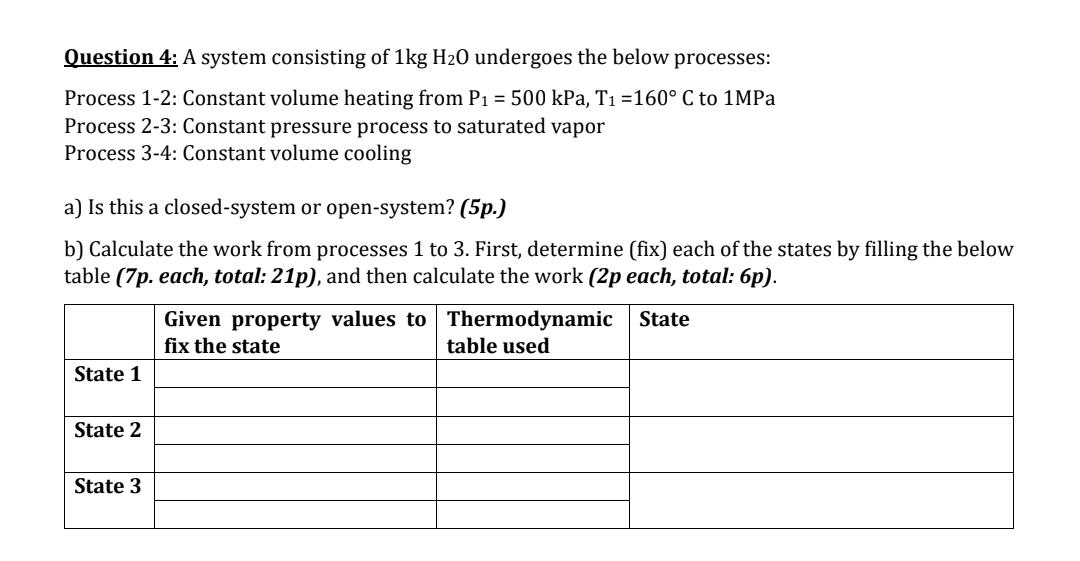
Question 4: A system consisting of 1kg H0 undergoes the below processes: Process 1-2: Constant volume heating from P = 500 kPa, T =160 C to 1MPa Process 2-3: Constant pressure process to saturated vapor Process 3-4: Constant volume cooling a) Is this a closed-system or open-system? (5p.) b) Calculate the work from processes 1 to 3. First, determine (fix) each of the states by filling the below table (7p. each, total: 21p), and then calculate the work (2p each, total: 6p). State 1 State 2 State 3 Given property values to Thermodynamic State table used fix the state
Step by Step Solution
3.55 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
a This system is a closedsystem because it consists of a fixed amount of water 1 kg undergoing var...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elements Of Chemical Reaction Engineering
Authors: H. Fogler
6th Edition
013548622X, 978-0135486221
Students also viewed these Mechanical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



