question 4
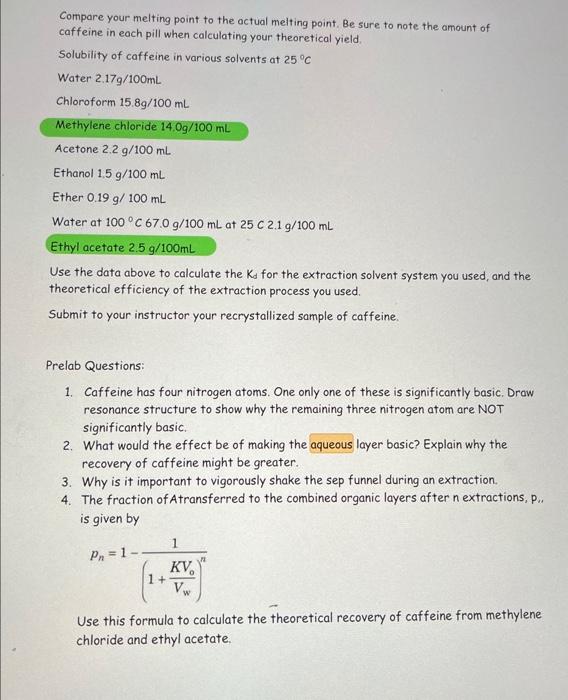
Compare your melting point to the actual melting point, Be sure to note the amount of caffeine in each pill when calculating your theoretical yield. Solubility of caffeine in various solvents at 25C Water 2.17g/100mL Chloroform 15.8g/100mL Methylene chloride 14.0g/100mL Acetone 2.2g/100mL Ethanol 1.5g/100mL Ether 0.19g/100mL Water at 100C67.0g/100mL at 25C2.1g/100mL Ethyl acetate 2.5g/100mL Use the data above to calculate the Kd for the extraction solvent system you used, and the theoretical efficiency of the extraction process you used. Submit to your instructor your recrystallized sample of caffeine. Prelab Questions: 1. Caffeine has four nitrogen atoms. One only one of these is significantly basic. Draw resonance structure to show why the remaining three nitrogen atom are NOT significantly basic. 2. What would the effect be of making the aqueous layer basic? Explain why the recovery of caffeine might be greater. 3. Why is it important to vigorously shake the sep funnel during an extraction. 4. The fraction of Atransferred to the combined organic layers after n extractions, p.. is given by pn=1(1+VwKVo)n1 Use this formula to calculate the theoretical recovery of caffeine from methylene chloride and ethyl acetate. Compare your melting point to the actual melting point, Be sure to note the amount of caffeine in each pill when calculating your theoretical yield. Solubility of caffeine in various solvents at 25C Water 2.17g/100mL Chloroform 15.8g/100mL Methylene chloride 14.0g/100mL Acetone 2.2g/100mL Ethanol 1.5g/100mL Ether 0.19g/100mL Water at 100C67.0g/100mL at 25C2.1g/100mL Ethyl acetate 2.5g/100mL Use the data above to calculate the Kd for the extraction solvent system you used, and the theoretical efficiency of the extraction process you used. Submit to your instructor your recrystallized sample of caffeine. Prelab Questions: 1. Caffeine has four nitrogen atoms. One only one of these is significantly basic. Draw resonance structure to show why the remaining three nitrogen atom are NOT significantly basic. 2. What would the effect be of making the aqueous layer basic? Explain why the recovery of caffeine might be greater. 3. Why is it important to vigorously shake the sep funnel during an extraction. 4. The fraction of Atransferred to the combined organic layers after n extractions, p.. is given by pn=1(1+VwKVo)n1 Use this formula to calculate the theoretical recovery of caffeine from methylene chloride and ethyl acetate








