Question
Question 5) Explain the relationship between the number of unpaired electrons and the change in mass that was observed. Be sure that this explanation is
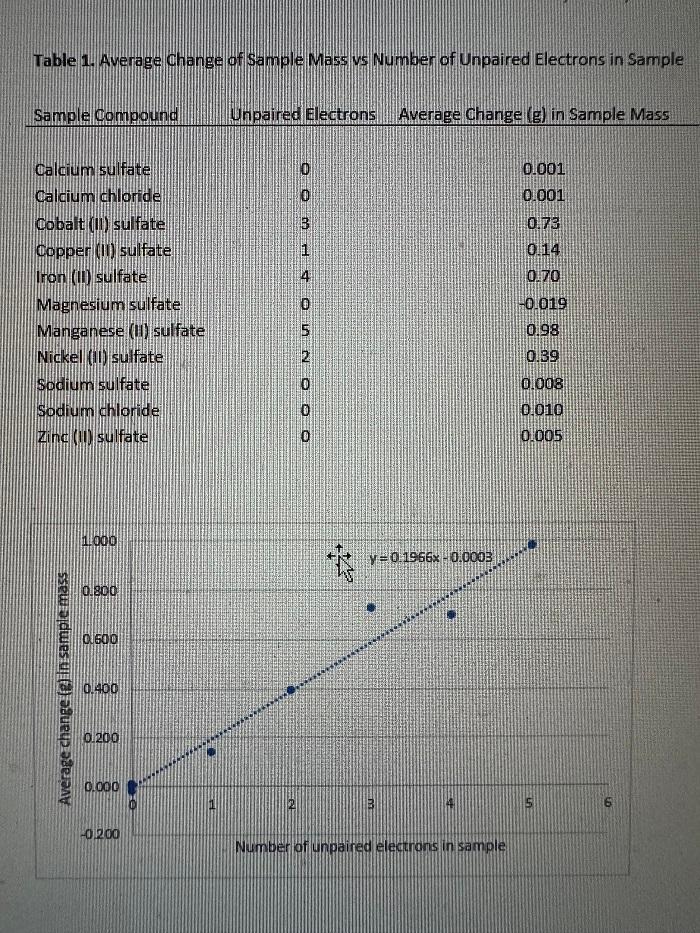
Question 5) Explain the relationship between the number of unpaired electrons and the change in mass that was observed. Be sure that this explanation is supported with evidence from the data collected.
About the question above, I feel like I have a grasp on the behavior of magnetism with diamagnetic (repulsion creates an increase in mass change) and paramagnetic substances (attraction creates a decrease in mass change), but my data is throwing me off. I was expecting to see, for example, magnesium sulfate to have a positive magnetic change since it has zero unpaired electrons - instead my data is showing a decrease in mass change, so it behaved as if it were paramagnetic. There are other paramagnetic compounds with a number of unpaired electrons, but the mass change was positive, not negative with a decreased weight. Am I misunderstanding something here or is my data off?
(Data was taken with a pocket balance and analytical balance so thats why you see different sig figs)
Table 1. Average Onange of sample Mass vs Number of Unpaired Electrons in SampleStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


