Answered step by step
Verified Expert Solution
Question
1 Approved Answer
QUESTION 5 Which of the following are good leaving groups? Choose all that apply. a. -OH b.-OTf c. -Br d.-OMs e. -Cl 1. -OTS

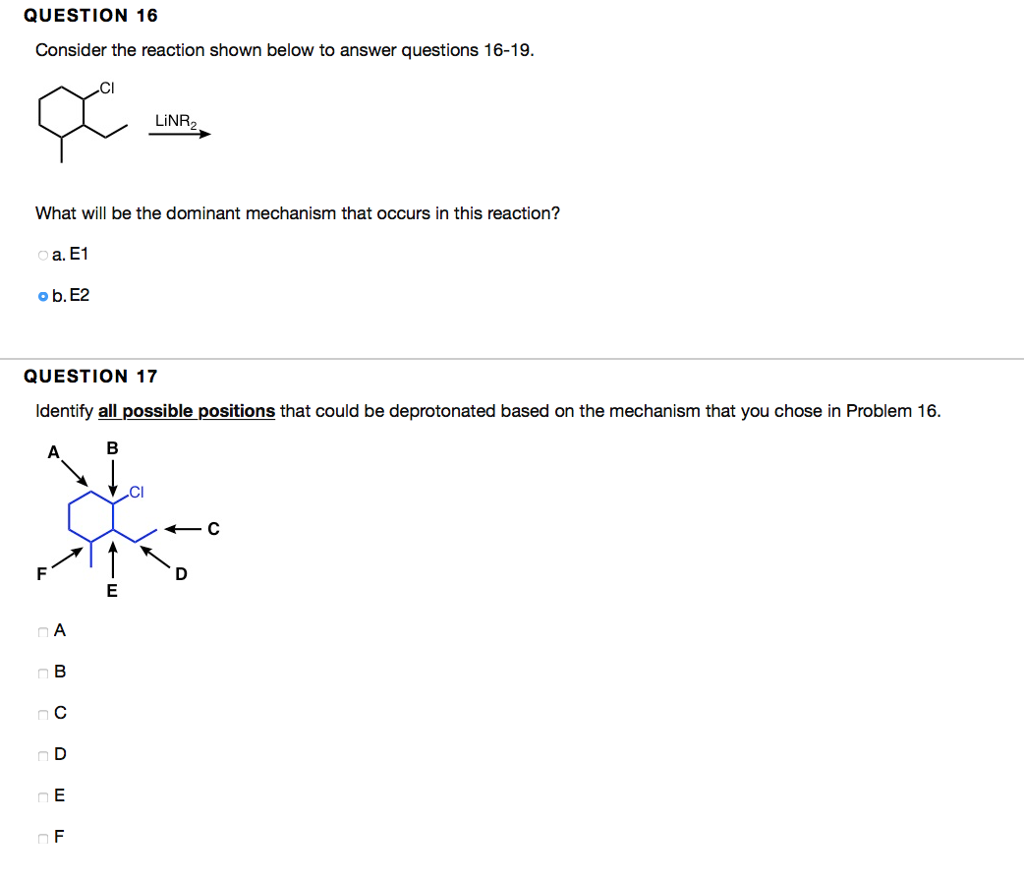
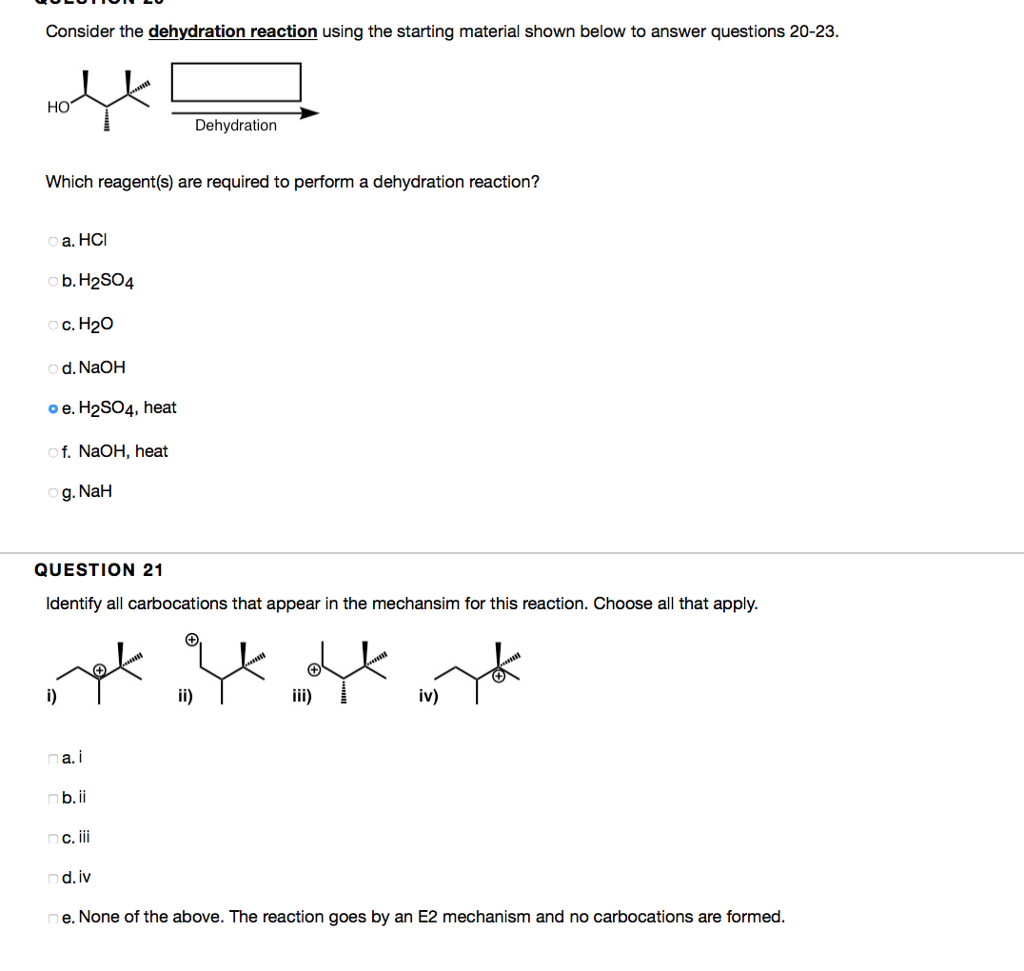
QUESTION 5 Which of the following are good leaving groups? Choose all that apply. a. -OH b.-OTf c. -Br d.-OMs e. -Cl 1. -OTS g.-F HO QUESTION 6 1 points Save Answer For problems 6-14, complete the reactions by filling the boxes with the necessary chemical reagent(s)/reaction conditions or major products. Provide reagent(s)/reaction conditions that would produce the major product most efficiently. Assume aqueous workups are performed if needed. OH a. Ag2O, NH4OH, HO b. NaBH4 c. CrO3, HCl, pyridine d. NaOH e, HBr f. HSO4 g. CrO3, HSO4, HO h. LIAIH4 oi. HO 0.25 points OH Save Answer QUESTION 16 Consider the reaction shown below to answer questions 16-19. x What will be the dominant mechanism that occurs in this reaction? oa. E1 ob. E2 QUESTION 17 Identify all possible positions that could be deprotonated based on the mechanism that you chose in Problem 16. B A A B C D E F A nc OD DE LINR OF E C D Consider the dehydration reaction using the starting material shown below to answer questions 20-23. Hoft HO Which reagent(s) are required to perform a dehydration reaction? oa. HCI ob. H2SO4 oc. HO od. NaOH o e. HSO4, heat of. NaOH, heat Og. NaH QUESTION 21 Identify all carbocations that appear in the mechansim for this reaction. Choose all that apply. i) na.i b.ii nc. iii Dehydration nd.iv ii) iii) iv) e. None of the above. The reaction goes by an E2 mechanism and no carbocations are formed.
Step by Step Solution
★★★★★
3.41 Rating (148 Votes )
There are 3 Steps involved in it
Step: 1
5 Ans a OH This is a poor leaving group because the hydroxide ion is a strong base and prefers to retain its electron pair b OTf tosylate This is a good leaving group because it is stable and can leav...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


