
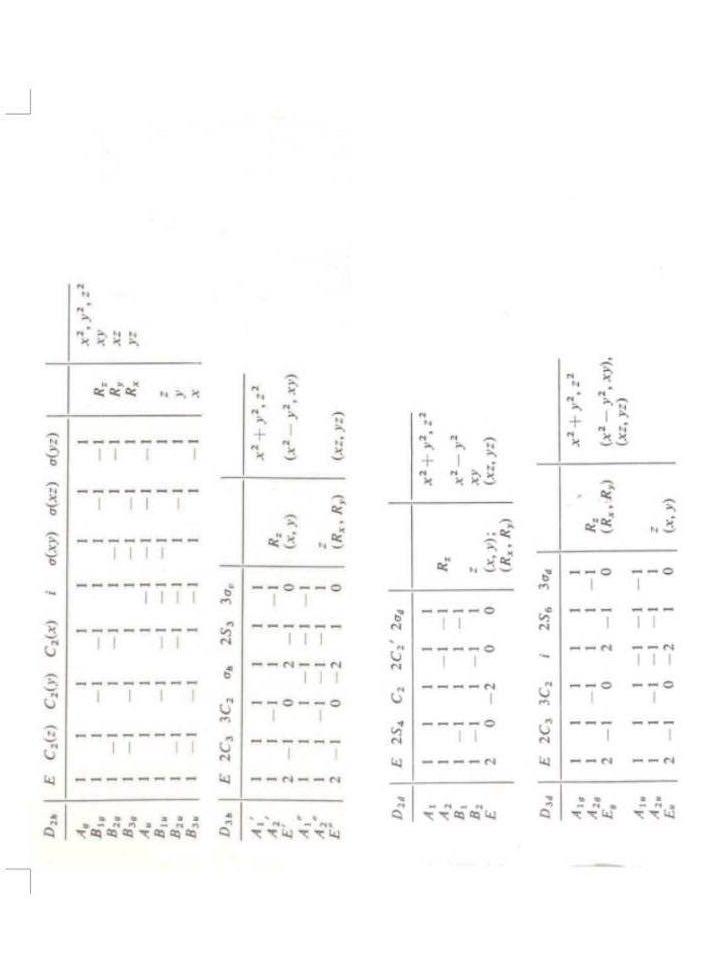
Question 6 (6 pts) We study the complexes (A)=[Co(OH2)6]2+ and (B)=[Co(Cl)4]2. The following figure presents the spectra of these two complexes in the visible domain. The spectra have been measured using an optical cuve with a optical pathway equal to 1cm and a concentration equal to 0,10molL1 for (A) et 1,7010 molL1 for (B). The magnetic moments are equal to 5,2B for (A) and 4,4B for (B). 1. Give the ground spectroscopic term of the free metal ion of these two complexes. 2. Using the magnetic data, determine if the complex (A) is high spin or low spin. 3. The complex (B) can exist with two different geometries: tetrahedral or planarsquare. Show that the determination of the magnetic moment allows to determine the good geometry of this complex 4. Deduce from the two previous questions the repartition of the d-electrons for these two complexes, and determine the ligand-field stabilization energy for each of the in function of 0. 5. Justify the large difference in intensity of the absorption bands of the two complexes. 6. The complex (A) is pale pink in color and the complex (B) is intense blue. Justify their respective colors. \begin{tabular}{l|rrrrrr|l|l} D3d & E & 2C3 & 3C2 & i & 2S6 & 3d & & \\ \hlineA1s & 1 & 1 & 1 & 1 & 1 & 1 & & \\ A2v & 1 & 1 & 1 & 1 & 1 & 1 & Rz & x2+y2,z2 \\ Ev & 2 & 1 & 0 & 2 & 1 & 0 & (Rx,Ry) & \\ (x2y2,xy),(xz,yz) \\ A1= & 1 & 1 & 1 & 1 & 1 & 1 & & \\ A2n & 1 & 1 & 1 & 1 & 1 & 1 & z & \\ Ev & 2 & 1 & 0 & 2 & 1 & 0 & (x,y) & \end{tabular} Question 6 (6 pts) We study the complexes (A)=[Co(OH2)6]2+ and (B)=[Co(Cl)4]2. The following figure presents the spectra of these two complexes in the visible domain. The spectra have been measured using an optical cuve with a optical pathway equal to 1cm and a concentration equal to 0,10molL1 for (A) et 1,7010 molL1 for (B). The magnetic moments are equal to 5,2B for (A) and 4,4B for (B). 1. Give the ground spectroscopic term of the free metal ion of these two complexes. 2. Using the magnetic data, determine if the complex (A) is high spin or low spin. 3. The complex (B) can exist with two different geometries: tetrahedral or planarsquare. Show that the determination of the magnetic moment allows to determine the good geometry of this complex 4. Deduce from the two previous questions the repartition of the d-electrons for these two complexes, and determine the ligand-field stabilization energy for each of the in function of 0. 5. Justify the large difference in intensity of the absorption bands of the two complexes. 6. The complex (A) is pale pink in color and the complex (B) is intense blue. Justify their respective colors. \begin{tabular}{l|rrrrrr|l|l} D3d & E & 2C3 & 3C2 & i & 2S6 & 3d & & \\ \hlineA1s & 1 & 1 & 1 & 1 & 1 & 1 & & \\ A2v & 1 & 1 & 1 & 1 & 1 & 1 & Rz & x2+y2,z2 \\ Ev & 2 & 1 & 0 & 2 & 1 & 0 & (Rx,Ry) & \\ (x2y2,xy),(xz,yz) \\ A1= & 1 & 1 & 1 & 1 & 1 & 1 & & \\ A2n & 1 & 1 & 1 & 1 & 1 & 1 & z & \\ Ev & 2 & 1 & 0 & 2 & 1 & 0 & (x,y) & \end{tabular}








