Question: Question 9 (2 points) Sucrose (table sugar) is hydrolyzed to glucose and fructose by the enzyme invertase. A Lineweaver-Burk plot (shown) was used to
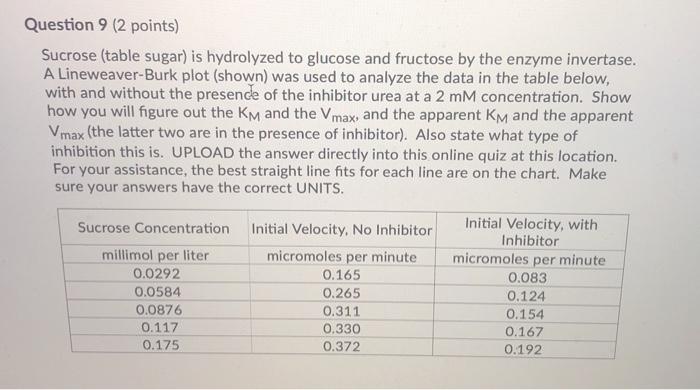
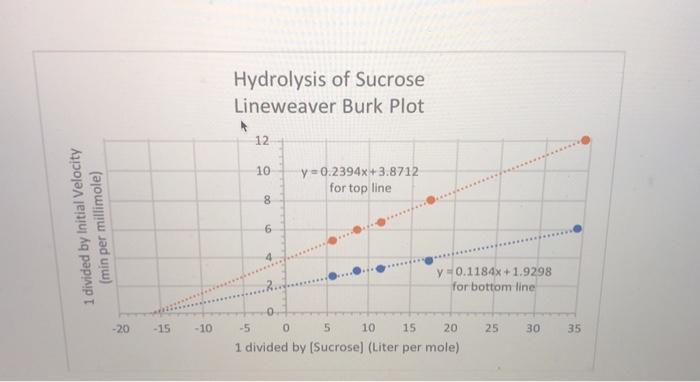
Question 9 (2 points) Sucrose (table sugar) is hydrolyzed to glucose and fructose by the enzyme invertase. A Lineweaver-Burk plot (shown) was used to analyze the data in the table below, with and without the presence of the inhibitor urea at a 2 mM concentration. Show how you will figure out the KM and the Vmax, and the apparent KM and the apparent Vmax (the latter two are in the presence of inhibitor). Also state what type of inhibition this is. UPLOAD the answer directly into this online quiz at this location. For your assistance, the best straight line fits for each line are on the chart. Make sure your answers have the correct UNITS. Sucrose Concentration millimol per liter 0.0292 0.0584 0.0876 0.117 0.175 Initial Velocity, No Inhibitor micromoles per minute 0.165 0.265 0.311 0.330 0.372 Initial Velocity, with Inhibitor micromoles per minute 0.083 0.124 0.154 0.167 0.192 1 divided by Initial Velocity (min per millimole) -20 -15 -10 Hydrolysis of Sucrose Lineweaver Burk Plot 12 10 8 6 y=0.2394x+3.8712 for top line y 0.1184x+1.9298 for bottom line -5 0 5 10 15 20 1 divided by [Sucrose] (Liter per mole). 25 30 35
Step by Step Solution
3.49 Rating (159 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


