Question
Given the following equation Znts) + 02e - ZnO(s) If 35.0 g of Zn is reacted with 70.0 g of O 2 - determine
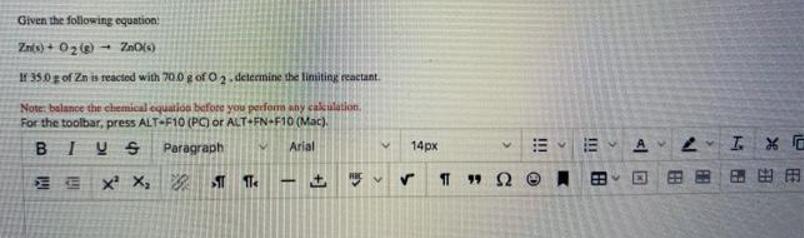
Given the following equation Znts) + 02e - ZnO(s) If 35.0 g of Zn is reacted with 70.0 g of O 2 - determine the liniting reactant. Note: balance the chemical equatioa before you perfom any cakulation. For the toolbar, press ALT-F10 (PC) or ALT+FN+F10 (Mac). BIUS Paragraph Arial 14px A E E X X, 99 2 !! !! +]
Step by Step Solution
3.44 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1
According to the law of conservati on of mass the balanced chemical equation must have the same numb...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: Joseph M. Hornback
2nd Edition
9781133384847, 9780199270293, 534389511, 1133384846, 978-0534389512
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



