Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Question) A gas undergoes a thermodynamic cycle consisting of three processes in series, beginning at state 1 following the provided data in the below
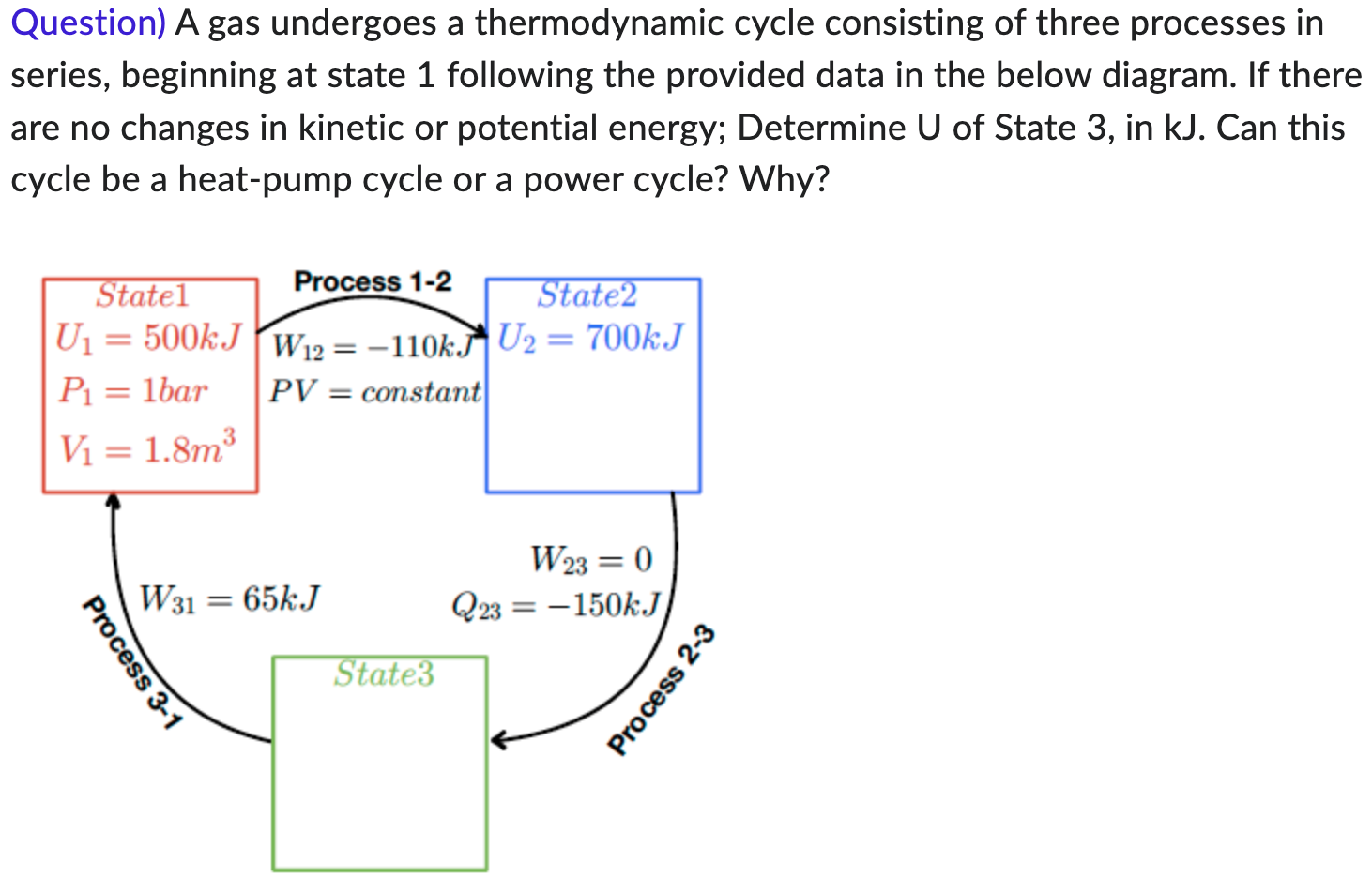
Question) A gas undergoes a thermodynamic cycle consisting of three processes in series, beginning at state 1 following the provided data in the below diagram. If there are no changes in kinetic or potential energy; Determine U of State 3, in kJ. Can this cycle be a heat-pump cycle or a power cycle? Why? Statel Process 1-2 State2 U = 500kJ W12=-110k. U2 = 700kJ P = 1bar PV = constant V = 1.8m W31 = 65kJ State3 Process 3-1 W23 = 0 Q23-150kJ Process 2-3
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


