Question from a Mass transport class. Textbook: Deen, Analysis of Transport Phenomena 2 edition Please answer all parts with a step-by-step solution. Thank you
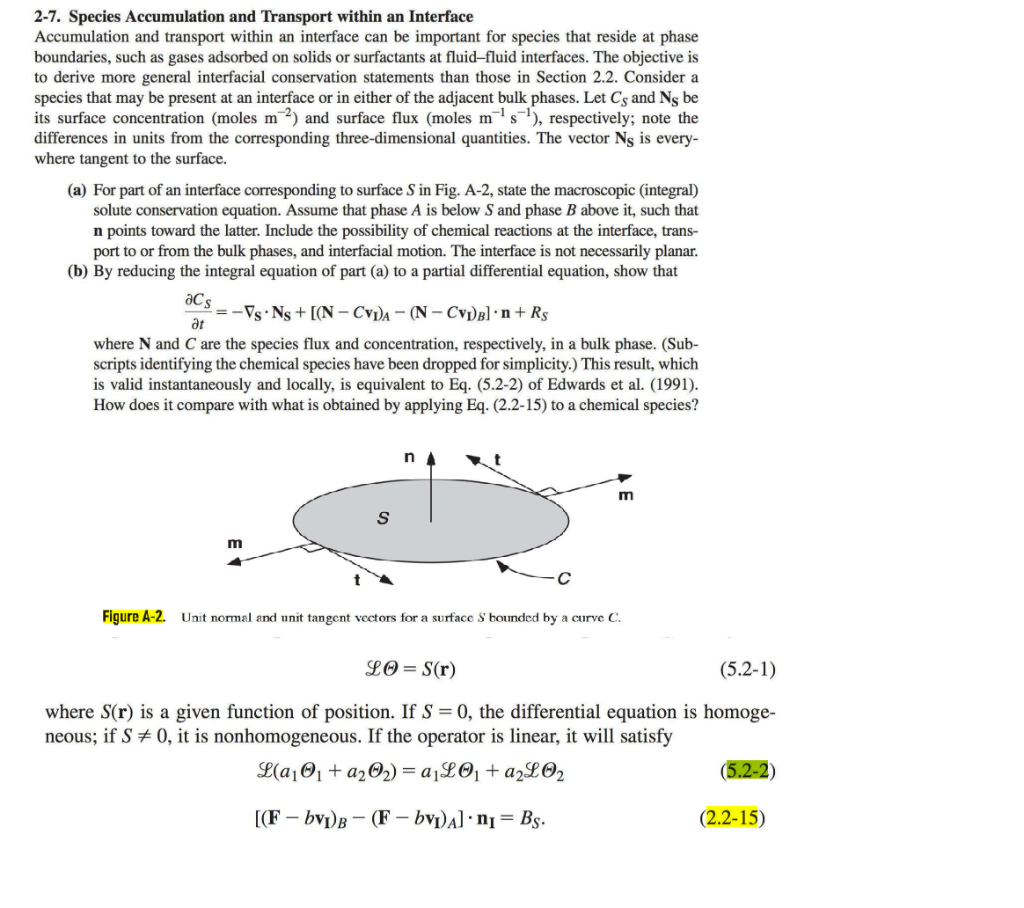
2-7. Species Accumulation and Transport within an Interface Accumulation and transport within an interface can be important for species that reside at phase boundaries, such as gases adsorbed on solids or surfactants at fluid-fluid interfaces. The objective is to derive more general interfacial conservation statements than those in Section 2.2. Consider a species that may be present at an interface or in either of the adjacent bulk phases. Let CS and NS be its surface concentration (moles m2 ) and surface flux (moles m1s1 ), respectively; note the differences in units from the corresponding three-dimensional quantities. The vector NS is everywhere tangent to the surface. (a) For part of an interface corresponding to surface S in Fig. A-2, state the macroscopic (integral) solute conservation equation. Assume that phase A is below S and phase B above it, such that n points toward the latter. Include the possibility of chemical reactions at the interface, transport to or from the bulk phases, and interfacial motion. The interface is not necessarily planar. (b) By reducing the integral equation of part (a) to a partial differential equation, show that tCS=SNS+[(NCvI)A(NCvI)B]n+RS where N and C are the species flux and concentration, respectively, in a bulk phase. (Subscripts identifying the chemical species have been dropped for simplicity.) This result, which is valid instantaneously and locally, is equivalent to Eq. (5.2-2) of Edwards et al. (1991). How does it compare with what is obtained by applying Eq. (2.215) to a chemical species? Figure A-2. Unit normal and unit tangent vectors for a surface S bounded by a curve C :. L=S(r) where S(r) is a given function of position. If S=0, the differential equation is homogeneous; if S=0, it is nonhomogeneous. If the operator is linear, it will satisfy L(a11+a22)=a1L1+a2L2[(FbvI)B(FbvI)A]nI=BS 2-7. Species Accumulation and Transport within an Interface Accumulation and transport within an interface can be important for species that reside at phase boundaries, such as gases adsorbed on solids or surfactants at fluid-fluid interfaces. The objective is to derive more general interfacial conservation statements than those in Section 2.2. Consider a species that may be present at an interface or in either of the adjacent bulk phases. Let CS and NS be its surface concentration (moles m2 ) and surface flux (moles m1s1 ), respectively; note the differences in units from the corresponding three-dimensional quantities. The vector NS is everywhere tangent to the surface. (a) For part of an interface corresponding to surface S in Fig. A-2, state the macroscopic (integral) solute conservation equation. Assume that phase A is below S and phase B above it, such that n points toward the latter. Include the possibility of chemical reactions at the interface, transport to or from the bulk phases, and interfacial motion. The interface is not necessarily planar. (b) By reducing the integral equation of part (a) to a partial differential equation, show that tCS=SNS+[(NCvI)A(NCvI)B]n+RS where N and C are the species flux and concentration, respectively, in a bulk phase. (Subscripts identifying the chemical species have been dropped for simplicity.) This result, which is valid instantaneously and locally, is equivalent to Eq. (5.2-2) of Edwards et al. (1991). How does it compare with what is obtained by applying Eq. (2.215) to a chemical species? Figure A-2. Unit normal and unit tangent vectors for a surface S bounded by a curve C :. L=S(r) where S(r) is a given function of position. If S=0, the differential equation is homogeneous; if S=0, it is nonhomogeneous. If the operator is linear, it will satisfy L(a11+a22)=a1L1+a2L2[(FbvI)B(FbvI)A]nI=BS







