Answered step by step
Verified Expert Solution
Question
1 Approved Answer
question from unimolecular decay topic please help to solve from chemical kinetics subject 4) Cyclopropane isomerization reaction is an unimolecular reaction. Experimental value of E*
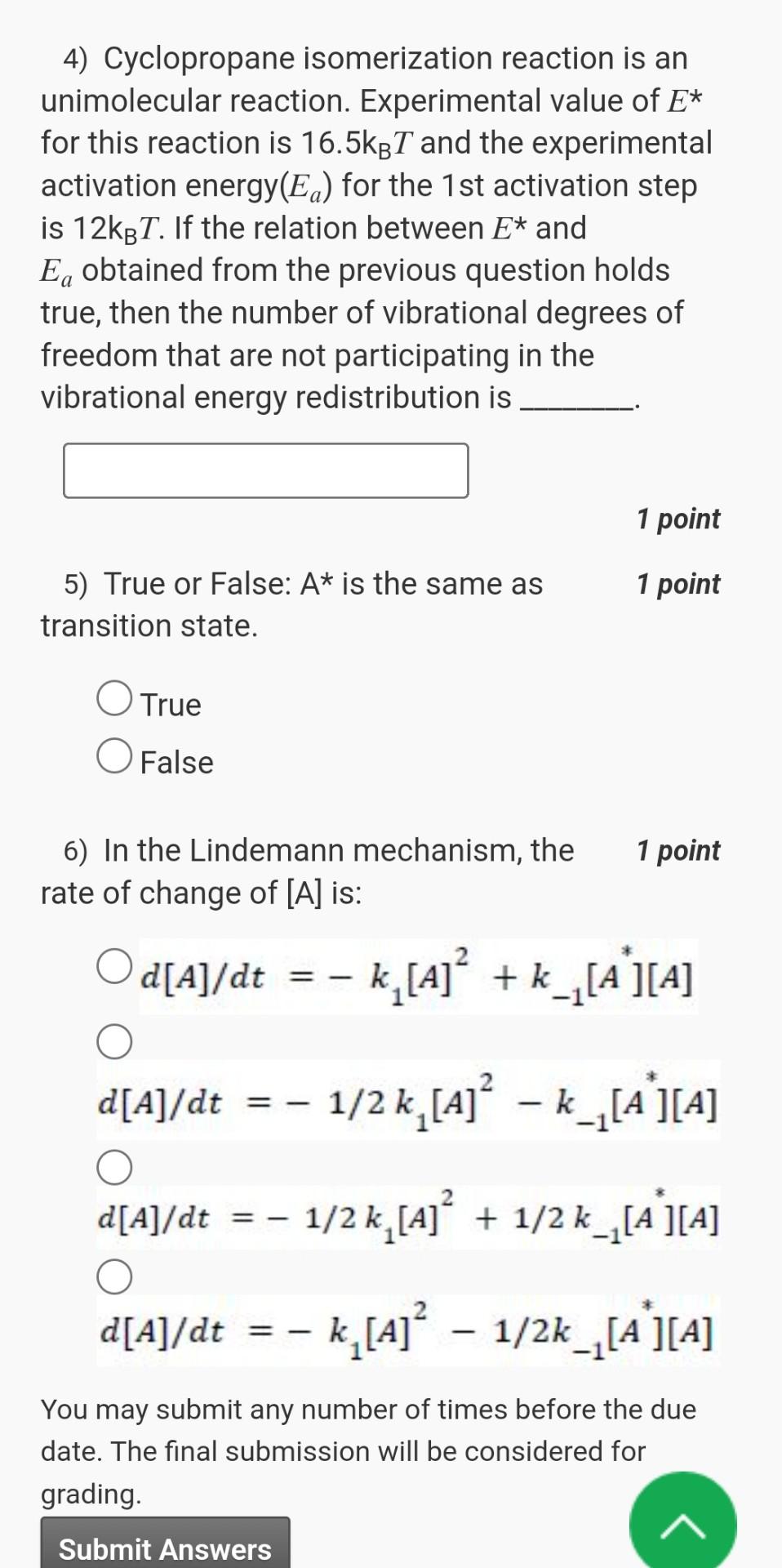
question from unimolecular decay topic please help to solve from chemical kinetics subject
4) Cyclopropane isomerization reaction is an unimolecular reaction. Experimental value of E* for this reaction is 16.5kBT and the experimental activation energy(Ea) for the 1 st activation step is 12kBT. If the relation between E* and Ea obtained from the previous question holds true, then the number of vibrational degrees of freedom that are not participating in the 1/2 kl[A] vibrational energy redistribution is 5) True or False: A* is the same as transition state. O True O False 6) In the Lindemann mechanism, the rate of change of [A] is: 1 point 1 point 1 point O O O d[AJ/dt = 2 = 1/2 kl[A] + 1/2 k 1 [A You may submit any number of times before the due date. The final submission will be considered for grading. Submit Answers
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


