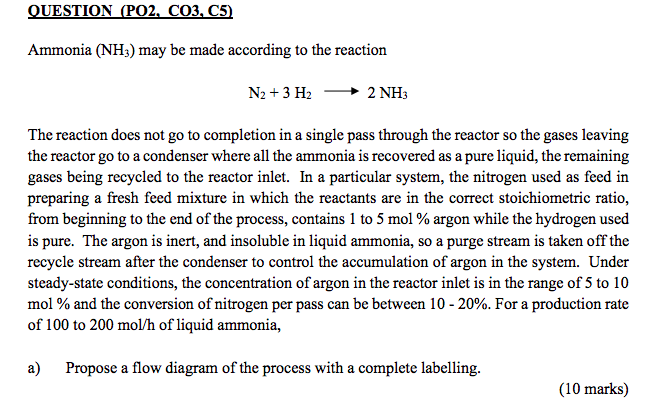

QUESTION (PO2, CO3, C5) Ammonia (NH3) may be made according to the reaction N2 + 3H2 2 NH The reaction does not go to completion in a single pass through the reactor so the gases leaving the reactor go to a condenser where all the ammonia is recovered as a pure liquid, the remaining gases being recycled to the reactor inlet. In a particular system, the nitrogen used as feed in preparing a fresh feed mixture in which the reactants are in the correct stoichiometric ratio, from beginning to the end of the process, contains 1 to 5 mol % argon while the hydrogen used is pure. The argon is inert, and insoluble in liquid ammonia, so a purge stream is taken off the recycle stream after the condenser to control the accumulation of argon in the system. Under steady-state conditions, the concentration of argon in the reactor inlet is in the range of 5 to 10 mol % and the conversion of nitrogen per pass can be between 10-20%. For a production rate of 100 to 200 mol/h of liquid ammonia, a) Propose a flow diagram of the process with a complete labelling. (10 marks) b) Solve the material balance of the system (molar flowrate of each stream and compositions of each component) and determine the overall conversion (in %) of nitrogen for the system. All assumption values must be in the range given. (20 marks) c) The liquid ammonia exits the condenser at temperature of -20C. Determine the pressure of the stream. Suggest three (3) suitable cooling medium used to achieve the purpose. Discuss your selection. Give your reference whenever required. (8 marks) d) The fresh feed of N2 and Argon are stored in a 100 L cylinder. The gas temperature is 43C. Determine the suitable pressure within the cylinder using: i) the ideal gas law. ii) the compressibility-factor equation of state. Compare the values obtained from both methods. Give your reference whenever required. (12 marks) QUESTION (PO2, CO3, C5) Ammonia (NH3) may be made according to the reaction N2 + 3H2 2 NH The reaction does not go to completion in a single pass through the reactor so the gases leaving the reactor go to a condenser where all the ammonia is recovered as a pure liquid, the remaining gases being recycled to the reactor inlet. In a particular system, the nitrogen used as feed in preparing a fresh feed mixture in which the reactants are in the correct stoichiometric ratio, from beginning to the end of the process, contains 1 to 5 mol % argon while the hydrogen used is pure. The argon is inert, and insoluble in liquid ammonia, so a purge stream is taken off the recycle stream after the condenser to control the accumulation of argon in the system. Under steady-state conditions, the concentration of argon in the reactor inlet is in the range of 5 to 10 mol % and the conversion of nitrogen per pass can be between 10-20%. For a production rate of 100 to 200 mol/h of liquid ammonia, a) Propose a flow diagram of the process with a complete labelling. (10 marks) b) Solve the material balance of the system (molar flowrate of each stream and compositions of each component) and determine the overall conversion (in %) of nitrogen for the system. All assumption values must be in the range given. (20 marks) c) The liquid ammonia exits the condenser at temperature of -20C. Determine the pressure of the stream. Suggest three (3) suitable cooling medium used to achieve the purpose. Discuss your selection. Give your reference whenever required. (8 marks) d) The fresh feed of N2 and Argon are stored in a 100 L cylinder. The gas temperature is 43C. Determine the suitable pressure within the cylinder using: i) the ideal gas law. ii) the compressibility-factor equation of state. Compare the values obtained from both methods. Give your reference whenever required. (12 marks)








