Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Question : The Joule-Thomson coefficient measures the change of temperature of a gas with respect to the change in pressure at constant enthalpy: B. (6
Question :
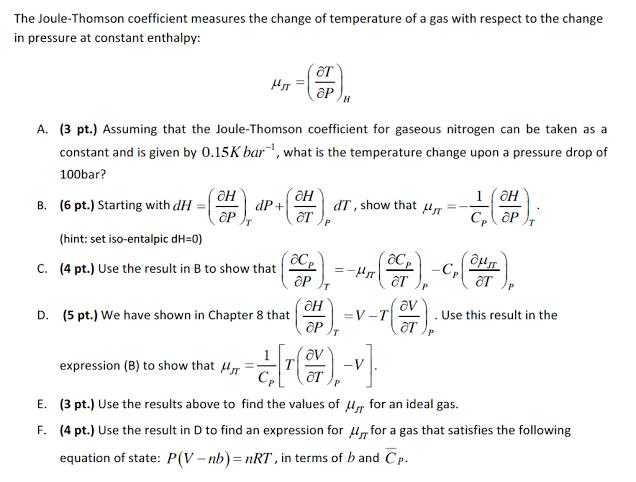
The Joule-Thomson coefficient measures the change of temperature of a gas with respect to the change in pressure at constant enthalpy: B. (6 pt.) Starting with dH = OH OP MJT A. (3 pt.) Assuming that the Joule-Thomson coefficient for gaseous nitrogen can be taken as a constant and is given by 0.15K bar, what is the temperature change upon a pressure drop of 100bar? T dP+ (hint: set iso-entalpic dH=0) C. (4 pt.) Use the result in B to show that OT ap D. (5 pt.) We have shown in Chapter 8 that H ar ace ap H H P dT, show that H =-H acp ar = =V-T -CP P 7 (27); " av 1 OH OP Cp T OT . Use this result in the OP T expression (B) to show that [-] E. (3 pt.) Use the results above to find the values of Mr for an ideal gas. F. (4 pt.) Use the result in D to find an expression for for a gas that satisfies the following equation of state: P(V-nb)=nRT, in terms of b and Cp.
Step by Step Solution
★★★★★
3.42 Rating (165 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


