Question
(Ch. 3.9) Given the balanced equation below, identify the limiting reactant when 0.251 mol of hydrogen react with 0.182 mol of oxygen. 2 H(g)
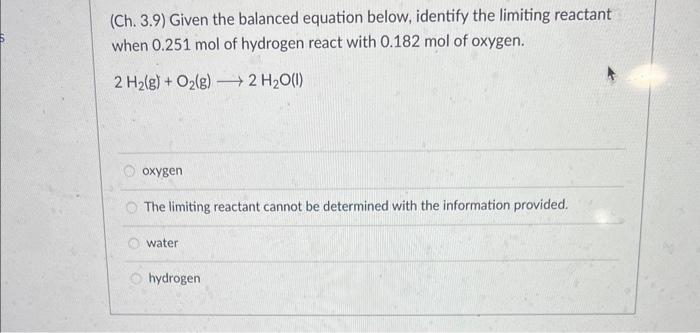
(Ch. 3.9) Given the balanced equation below, identify the limiting reactant when 0.251 mol of hydrogen react with 0.182 mol of oxygen. 2 H(g) + O(g) 2 HO(l) oxygen The limiting reactant cannot be determined with the information provided. water hydrogen
Step by Step Solution
3.42 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1
2H 0 2HO reactant used 0251mole H 0182 mole 0 2 mol H need...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Probability And Statistics
Authors: Morris H. DeGroot, Mark J. Schervish
4th Edition
9579701075, 321500466, 978-0176861117, 176861114, 978-0134995472, 978-0321500465
Students also viewed these Mathematics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



