Question
The method of internal normalization was chosen to determine the mass of a sample comprising a mixture of four esters of butanoic acids. To
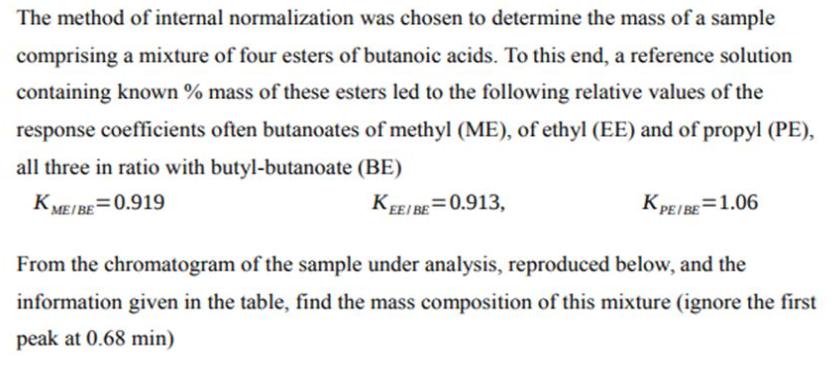
The method of internal normalization was chosen to determine the mass of a sample comprising a mixture of four esters of butanoic acids. To this end, a reference solution containing known % mass of these esters led to the following relative values of the response coefficients often butanoates of methyl (ME), of ethyl (EE) and of propyl (PE), all three in ratio with butyl-butanoate (BE) KME/BE=0.919 KEE/BE=0.913, KPE/BE=1.06 From the chromatogram of the sample under analysis, reproduced below, and the information given in the table, find the mass composition of this mixture (ignore the first peak at 0.68 min)
Step by Step Solution
3.33 Rating (147 Votes )
There are 3 Steps involved in it
Step: 1
The system of internal normalization is used to determine the relative composition of a admixture of ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Introduction to Management Accounting
Authors: Charles Horngren, Gary Sundem, Jeff Schatzberg, Dave Burgsta
16th edition
978-0133058819, 9780133059748, 133058816, 133058786, 013305974X , 978-0133058789
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



