Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2. Carnot Engine A uses 1 mol monatomic ideal gas as working medium. The temperature for isothermal expansion is 47o. The temperature for isothermal
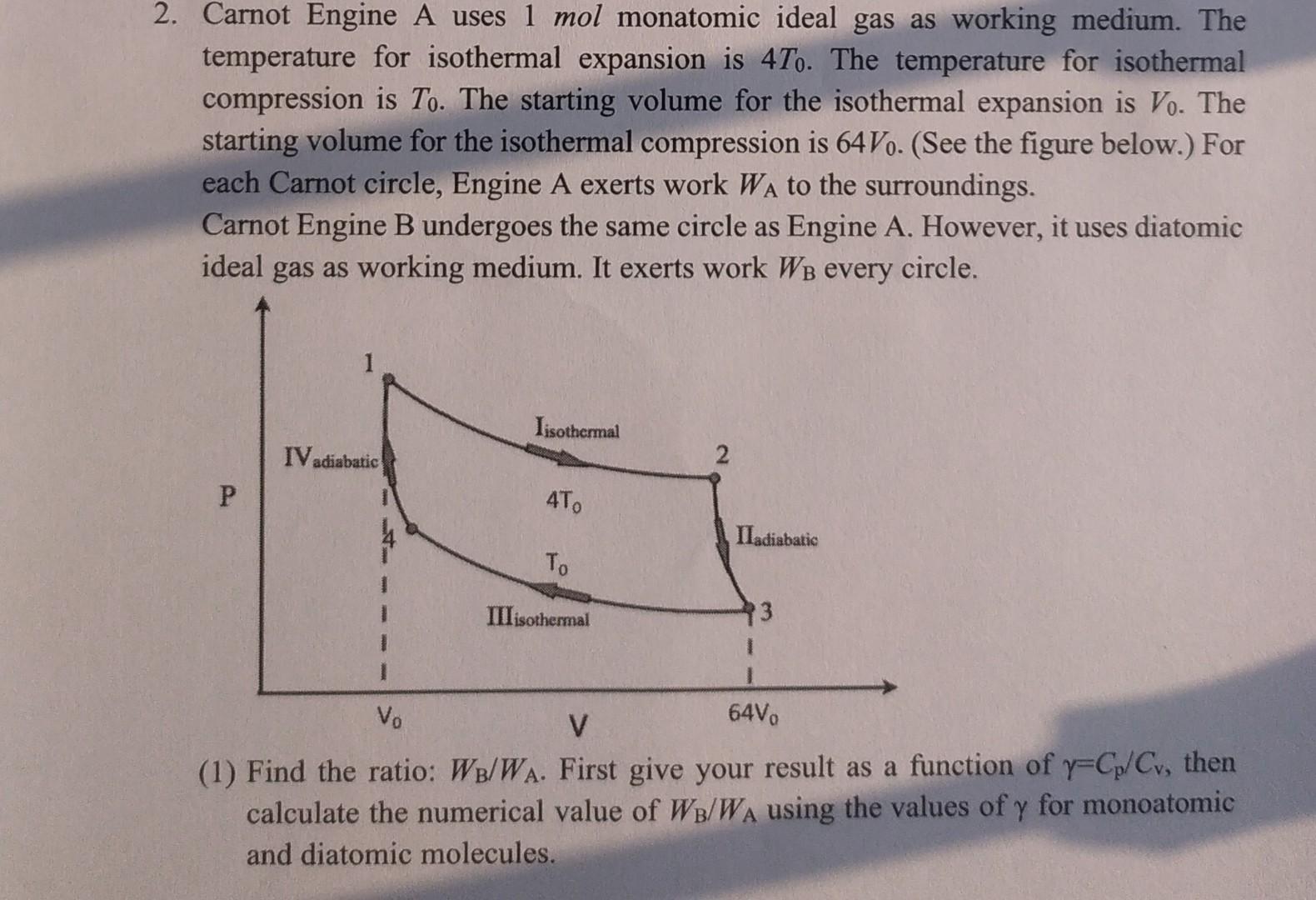
2. Carnot Engine A uses 1 mol monatomic ideal gas as working medium. The temperature for isothermal expansion is 47o. The temperature for isothermal compression is To. The starting volume for the isothermal expansion is Vo. The starting volume for the isothermal compression is 64Vo. (See the figure below.) For each Carnot circle, Engine A exerts work WA to the surroundings. Carnot Engine B undergoes the same circle as Engine A. However, it uses diatomic ideal gas as working medium. It exerts work W every circle. P IV adiabatic Iisothermal 4To To III isothermal 2 IIadiabatic 13 Vo 64Vo V (1) Find the ratio: WB/WA. First give your result as a function of y-Cp/Cv, then calculate the numerical value of WB/WA using the values of y for monoatomic and diatomic molecules.
Step by Step Solution
★★★★★
3.41 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


