Question
(b) (i) (ii) Deduce whether the equation given below is thermodynamically consistent X = mole fraction Derive an equation for dependence of enthalpy change
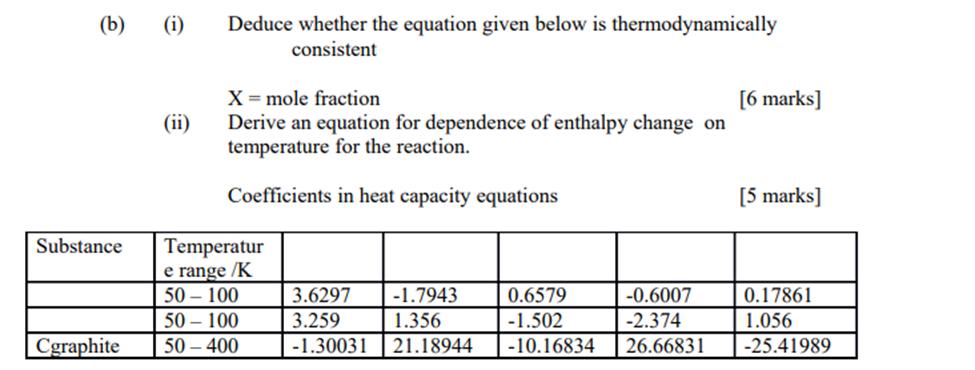
(b) (i) (ii) Deduce whether the equation given below is thermodynamically consistent X = mole fraction Derive an equation for dependence of enthalpy change on temperature for the reaction. Coefficients in heat capacity equations Substance Temperatur e range /K 50-100 50-100 Cgraphite 50-400 3.6297 -1.7943 3.259 1.356 -1.30031 21.18944 0.6579 -1.502 -10.16834 -0.6007 -2.374 26.66831 [6 marks] [5 marks] 0.17861 1.056 -25.41989
Step by Step Solution
3.48 Rating (148 Votes )
There are 3 Steps involved in it
Step: 1
bi Deduce whether the equation given below is thermodynamically consistent X mole fraction Solution The equation X mole fraction is indeed thermodynam...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elementary Principles of Chemical Processes
Authors: Richard M. Felder, Ronald W. Rousseau
3rd Edition
978-0471687573, 9788126515820, 978-0-471-4152, 0471720631, 047168757X, 8126515821, 978-0471720638
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



