Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(b) Phosphorus in urine can be determined by treating with molybdenum (VI) and then reducing the phosphomolybdatewith aminaphtholsulfonic acid to give the characteristic molybdenum

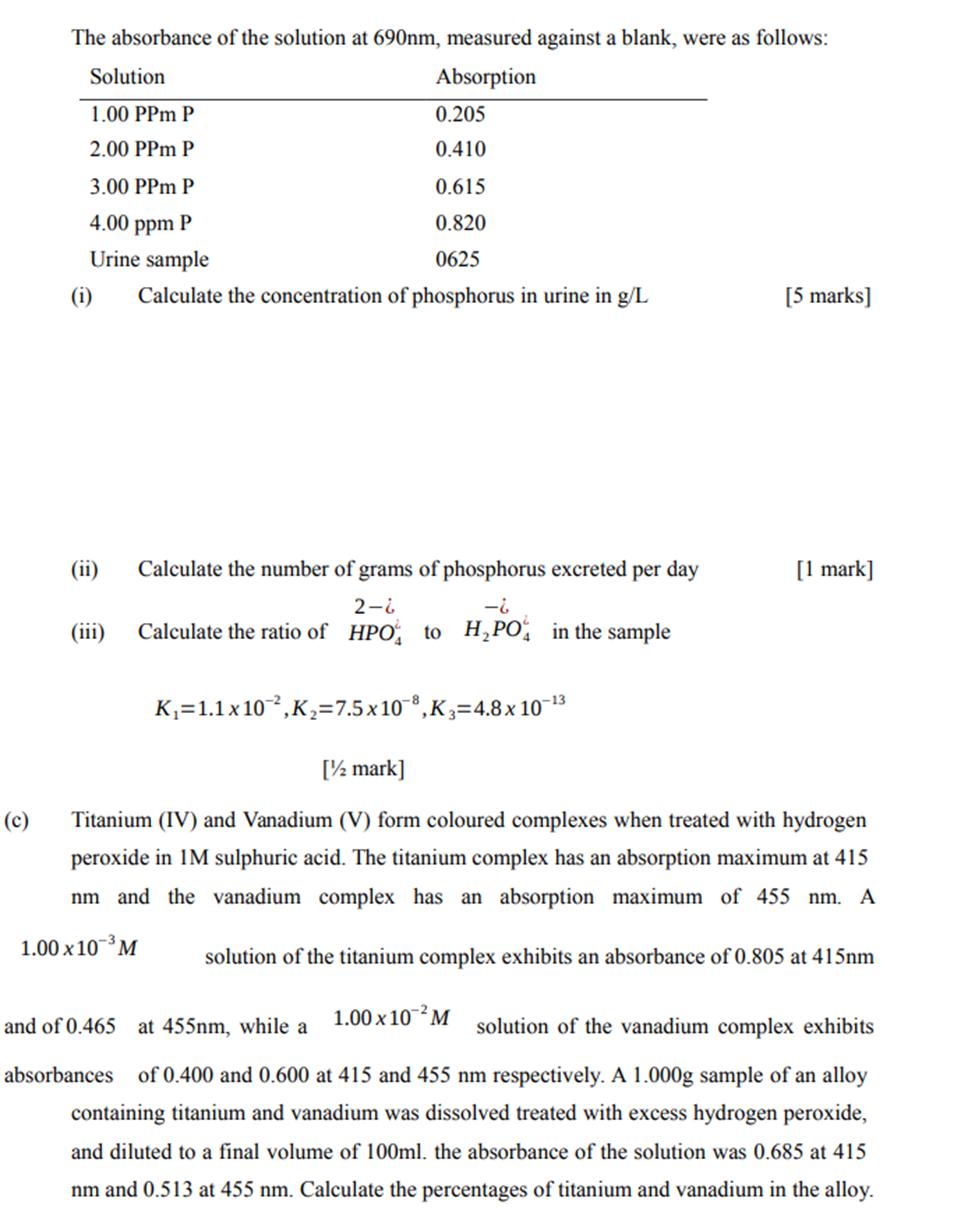
(b) Phosphorus in urine can be determined by treating with molybdenum (VI) and then reducing the phosphomolybdatewith aminaphtholsulfonic acid to give the characteristic molybdenum blue colour. This absorbs at 690 nm. A patient excreted 1270 ml urine in 24h and the pH of the urine was 6.5. A 1.00 ml a aliquot of the urine was treated with molybdate reagent and aminophtholsulfonic acid and was diluted to a volume of 50.0ml. A series of phosphate standards was similarly treated. The absorbance of the solution at 690nm, measured against a blank, were as follows: Absorption Solution 1.00 PPM P 0.205 2.00 PPM P 0.410 3.00 PPM P 0.615 4.00 ppm P 0.820 Urine sample 0625 Calculate the concentration of phosphorus in urine in g/L (i) (ii) (iii) Calculate the number of grams of phosphorus excreted per day 2-6 -6 Calculate the ratio of HPO to HPO4 in the sample K=1.1 x 102, K=7.5 x 108, K3=4.8 x 10-13 [5 marks] [1 mark] [ mark] Titanium (IV) and Vanadium (V) form coloured complexes when treated with hydrogen peroxide in 1M sulphuric acid. The titanium complex has an absorption maximum at 415 nm and the vanadium complex has an absorption maximum of 455 nm. A 1.00 x 10 M solution of the titanium complex exhibits an absorbance of 0.805 at 415nm and of 0.465 at 455nm, while a 1.00 x 10-M solution of the vanadium complex exhibits absorbances of 0.400 and 0.600 at 415 and 455 nm respectively. A 1.000g sample of an alloy containing titanium and vanadium was dissolved treated with excess hydrogen peroxide, and diluted to a final volume of 100ml. the absorbance of the solution was 0.685 at 415 nm and 0.513 at 455 nm. Calculate the percentages of titanium and vanadium in the alloy.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
i To calculate the concentration of phosphorus in urine in gL we can use the BeerLambert law which states that the absorbance A of a solution is direc...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


