Answered step by step
Verified Expert Solution
Question
1 Approved Answer
questions 4-10 Be sure all the columns of Table 2 are filled in. Identify where apli... occurs in Titration Region 1 and where ApH... occurs
questions 4-10 
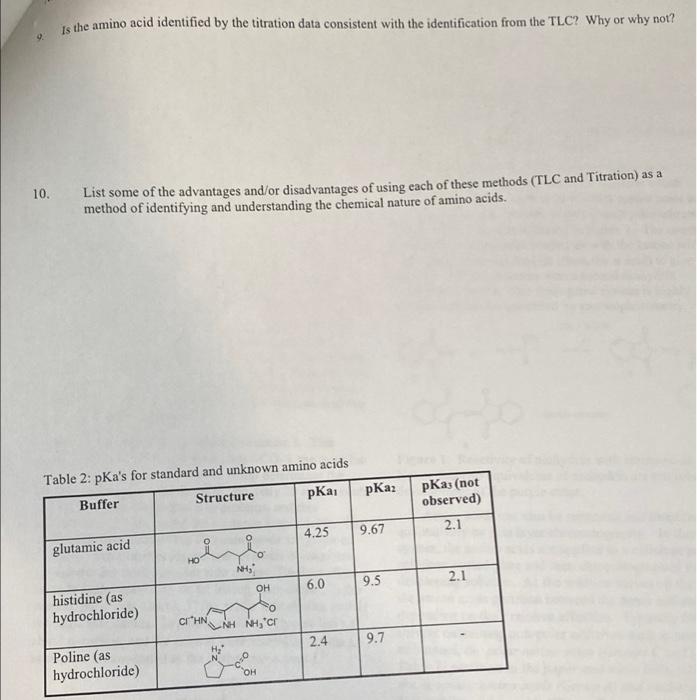
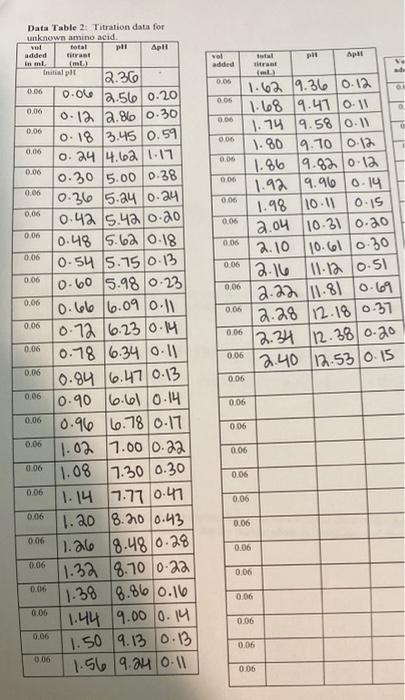
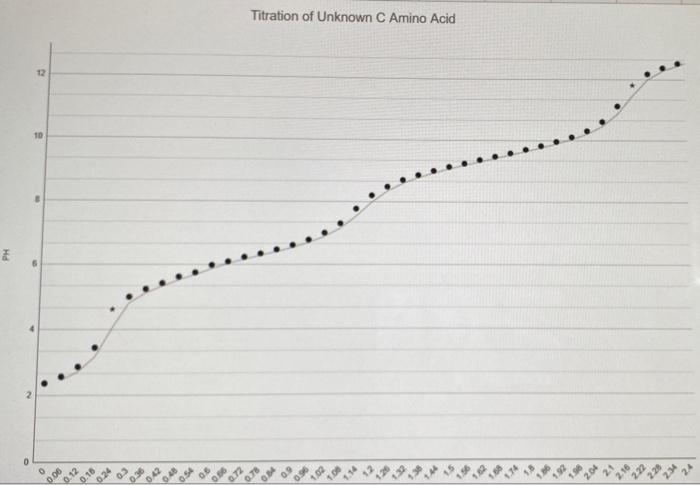
Be sure all the columns of Table 2 are filled in. Identify where apli... occurs in Titration Region 1 and where ApH... occurs in Titration Region 2 ApH... Titration Region 1 ApH... Titration Region 2 Using your data in Table 2, and the results given in the question above, construct a graph of the titration curve of the unknown amino acid. Put the pH on the Yaxis and the total volume of titrant added on the Xaxis Connect consecutive points with a smooth line. Identify the two endpoints and the two pka's from the titration curve data. Draw lines on the graphs indicating these volumes and related pls. 6. Fill in the table below using your graph. parameter Titration region 1 Titration region 2 Endpoint volume pH pka 7 Consider your answers in the table above. How did you determine the pH in each region? How is the pH related to the pKa in cach Titration Region 8. A list of amino acids and their respective pka's are given below. Indicate which no more than one) of the amino acids has pKa's which are most consistent with the pka's of the unknown amino acid you determined in the AA titration Is the amino acid identified by the titration data consistent with the identification from the TLC? Why or why not? o 10. List some of the advantages and/or disadvantages of using each of these methods (TLC and Titration) as a method of identifying and understanding the chemical nature of amino acids. Table 2: pKa's for standard and unknown amino acids Buffer Structure pKai pKaz pKas (not observed) 2.1 4.25 9.67 O glutamic acid NH 9.5 2.1 6.0 OH histidine (as hydrochloride) cr HN NH NH, Cr 2.4 9.7 Poline (as hydrochloride) total #t Apli Data Table 2: Titration data for unknown amino acid. total pl Apl added tirant in ml Trifalt 2.30 0.00 0.0 2.56 0.20 0.06 ol added 0.06 0.06 0 0.06 D 0.06 0.06 0,06 0.06 0.06 0.06 0.06 0.06 0.06 0.06 ml 1.62 9.36 0.12 1.68 9.410.11 1.74 9.58 0.1 1.80 9.10 0.12 1.86 9.82 0.12 1.929.96 0.14 1.98 10.11 0.15 2.04 10.31 0.20 2.10 10.610.30 2.16 11.12 0.51 2.22 11.81 0.69 2.28 /12.18/0.37 12.34 12.380.20 12.40 12.53/0.15 0.06 0.06 0.06 0.06 0.06 0.06 0.06 0.06 0.06 0.06 0.06 0.06 0.06 0.06 0.12 2.86 0:30 0.18 3.45 0.59 0 24 4.62 1.17 0.30 5.00 0.38 10.36 5.24 0.24 0.42 $.40 0.20 10.48 5.62 0.18 0.54 5.75 0-13 0.60 5.98 0.23 0.66 6.09 0.11 10.72 6.23/0.14 0.78 6.34 0.11 10.84 6.47 0.13 10.90 6.610.14 0.96 6.78 0.17 1.02 .000.22 11.08 7.30 0.30 0.06 1.14 7.770.47 0.06 1.20 8.200.43 (1.266 8.480.28 1.32 8.700.22 1.38 8.86 0.16 1.44 9.000.14 0.06 1.50 9.13 0.3 1.56 9.24 10-11 0.06 0.06 0.06 0.06 0.06 0.06 0.06 0.06 0.06 0.06 0.06 0.06 0.06 0.06 0.06 0.06 0.06 0.06 0.06 0.06 0.06 Titration of Unknown C Amino Acid 12 10 PH 6 



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


