Answered step by step
Verified Expert Solution
Question
1 Approved Answer
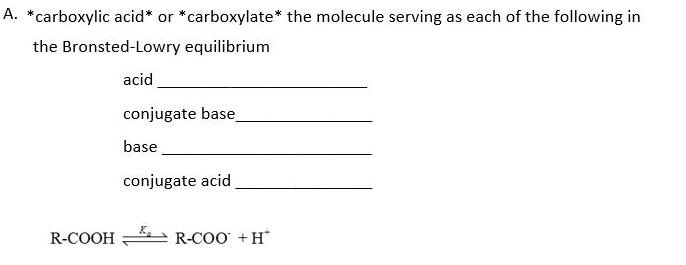
A. *carboxylic acid* or *carboxylate* the molecule serving as each of the following in the Bronsted-Lowry equilibrium acid conjugate base base conjugate acid R-COOH
![3. How does the [R-COO] /[R-COOH] compare with 1, if the pH is 7.4? 4. The total of all R molecules in solution may be expres](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2021/06/60b7b88556f2b_1622653058944.jpg)
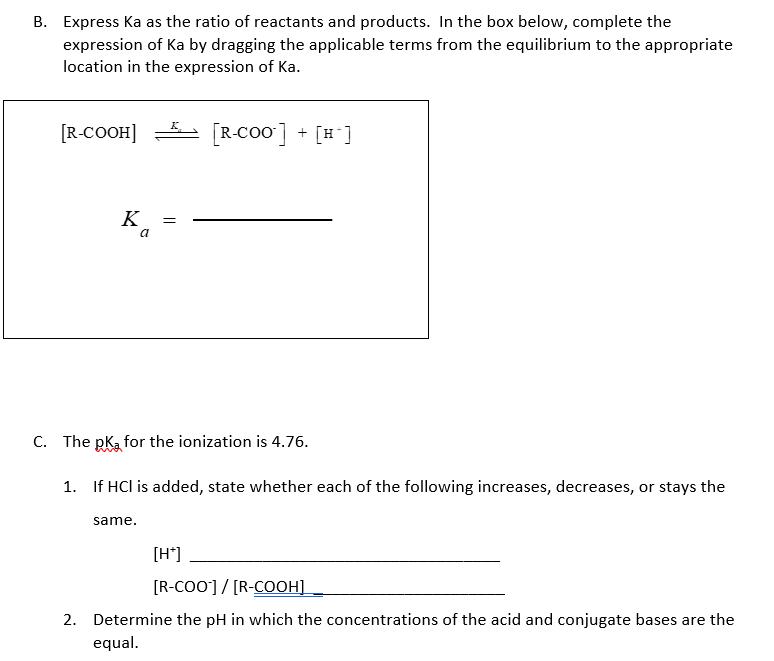
A. *carboxylic acid* or *carboxylate* the molecule serving as each of the following in the Bronsted-Lowry equilibrium acid conjugate base base conjugate acid R-COOH R-COO +H* B. Express Ka as the ratio of reactants and products. In the box below, complete the expression of Ka by dragging the applicable terms from the equilibrium to the appropriate location in the expression of Ka. [R-COOH] [R-Coo] + [H ] K = a C. The pka for the ionization is 4.76. 1. If HCl is added, state whether each of the following increases, decreases, or stays the same. [H*] [R-CO0]/ [R-COOH] 2. Determine the pH in which the concentrations of the acid and conjugate bases are the equal. 3. How does the [R-COO] / [R-COOH] compare with 1, if the pH is 7.4? 4. The total of all R molecules in solution may be expressed as [R], is [R] = [R-COO] + [R-COOH]. Which type of functional group is most abundant among R molecules based on the following? lonized or neutral (no charge). Base or acid
Step by Step Solution
★★★★★
3.59 Rating (163 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


